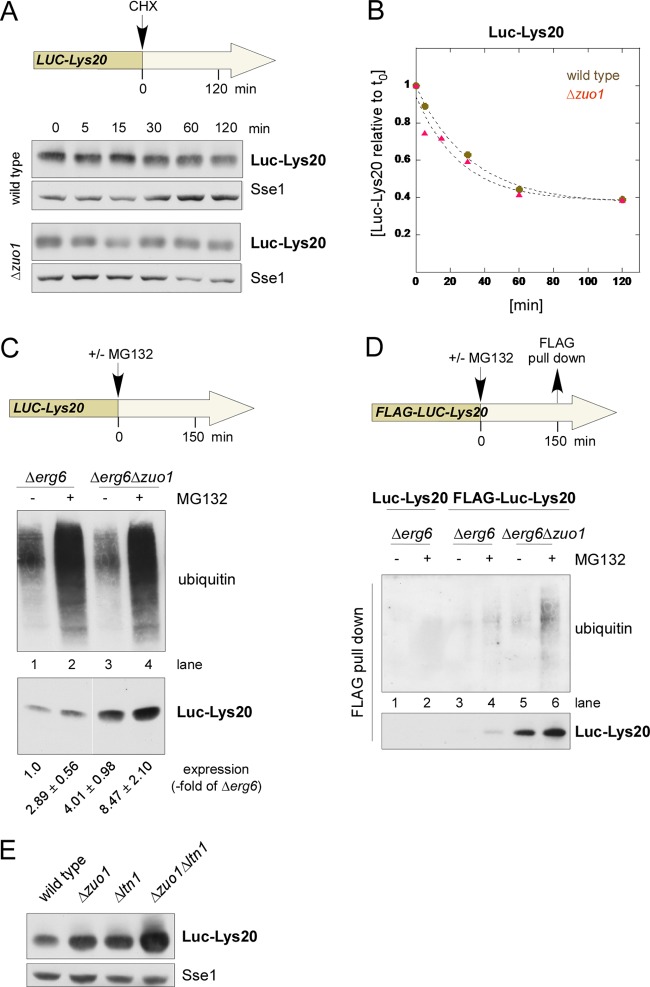
Fig 4.
RAC/Ssb acts independently of the proteolytic machinery. (A) Stability of Luc-Lys20 in wild-type and Δzuo1 strains. De novo protein synthesis of logarithmically growing cultures was inhibited by the addition of cycloheximide (CHX; final concentration, 100 μg/ml). The level of Luc-Lys20 expression was monitored at the time points indicated. Total cell extracts were analyzed via immunoblotting using antibodies directed against luciferase and Sse1. (B) Half-life of Luc-Lys20 in wild-type and Δzuo1 strains. Quantification of the data shown in panel A. The intensity of the Luc-Lys20 band was normalized for differences in loading using the intensity of the Sse1 band. The intensity at time zero (t0) was set equal to 1, and the data were fitted to an exponential decay function. (C) Expression of Luc-Lys20 in the Δerg6 and Δerg6 Δzuo1 strains after proteasome inhibition. The proteasome inhibitor MG132 was added to logarithmically growing cultures as indicated. Accumulation of polyubiquitinated proteins was monitored via immunoblotting using ubiquitin-specific antibody. The level of Luc-Lys20 expression was analyzed via immunoblotting using luciferase antibody. Expression of Luc-Lys20 was quantified in three independent experiments. Mean values and the SEM relative to untreated Δerg6 (lane 1) are given below a representative immunoblot. (D) Polyubiquitination of FLAG-Luc-Lys20 in Δerg6 and Δerg6 Δzuo1 strains. Total extracts of Δerg6 and Δerg6 Δzuo1 strains expressing Luc-Lys20 or FLAG-Luc-Lys20, as indicated, were subjected to affinity purification using FLAG-beads. Polyubiquitination of affinity-purified FLAG-Luc-Lys20 was analyzed via immunoblotting using ubiquitin-specific antibody (ubiquitin). The amount of isolated FLAG-Luc-Lys20 was determined using an antibody directed against luciferase. (E) Expression level of Luc-Lys20 in wild-type, Δzuo1, Δltn1, and Δzuo1 Δltn1 strains. Total cell extracts were analyzed via immunoblotting using antibodies specific for luciferase and, as a loading control, Sse1.