Fig 2.
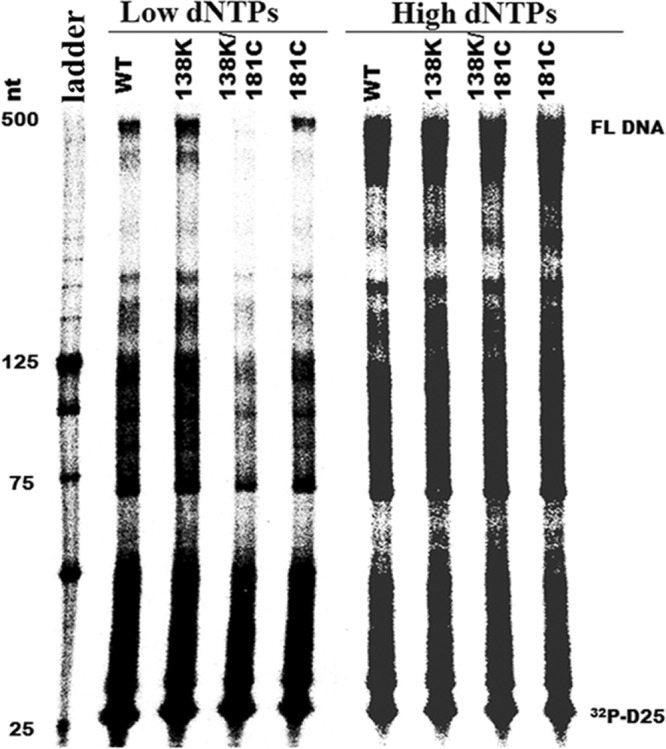
The Y181C mutation in HIV-1 RT diminishes enzyme processivity of E138K-containing enzymes at low dNTP concentrations. The processivity of purified recombinant RT enzymes was analyzed using 5′ end-labeled DNA primer (D25) annealed to an HIV-1 PBS RNA template as the substrate; the resulting full-length DNA (FL DNA) is 471 nt in size. Processivities were determined by the size distribution of DNA products in fixed-time experiments at two different concentrations of dNTPs in the presence of heparin trap. (A) Low dNTP concentration (0.5 μM). (B) High dNTP concentration (200 μM). Sizes of some fragments of the 32P-labeled 25-bp DNA ladder (Invitrogen, Burlington, ON, Canada) in nucleotide bases are indicated on the left side of the panel. All reaction products were resolved by denaturing 6% polyacrylamide gel electrophoresis and visualized by phosphorimaging. Positions of [32P]-labeled D25 primer (32P-D25) and the 471-nt full-length extension DNA (FL DNA) product are indicated on the right.
