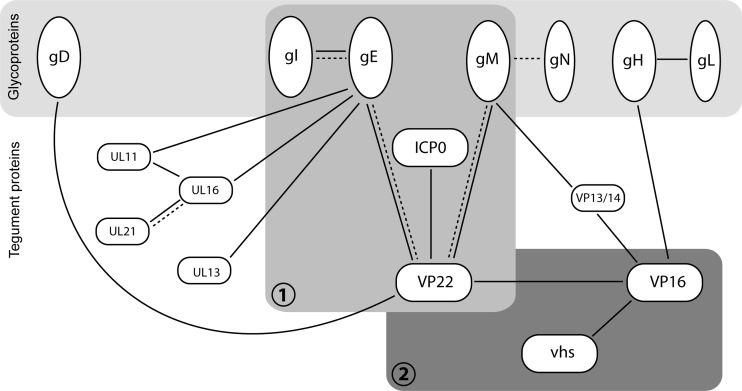
Fig 8.
Network of protein-protein interactions around the HSV-1 tegument protein VP22. Solid lines indicate interactions reported in HSV-1 (5, 8, 12, 20, 22, 23, 26, 34, 38, 42, 48–50, 52, 53). Broken lines indicate interactions shown in PRV (19, 27, 28, 55). The glycoprotein-tegument complex characterized here (complex 1), which is required for VP22 and ICP0 assembly into the virion, is shown in the context of previously reported relevant interactions (19, 38, 49). Whether UL11, UL16, or UL13 are incorporated into the VP22-gE-gM-gI-ICP0 complex via their known interactions with gE remains to be determined (22, 42, 53). Others have observed VP22-gD binding in infected cells (5, 17); however, this interaction was not reproducible in our own hands (49), and gD was not detectable in the VP22-gE-gM-gI-ICP0 complex. The previously characterized VP22-VP16 complex, which also incorporates vhs (12, 48, 50) and which was shown here to be separate from the VP22 assembly complex, is also illustrated (complex 2). For completion, the tegument protein VP13/14 has also been included in the figure. VP13/14 has been shown to interact with the cytoplasmic tail of gM (49) and VP16 (8, 52); however, it is not known which if either of these interactions is involved in VP13/14 assembly.