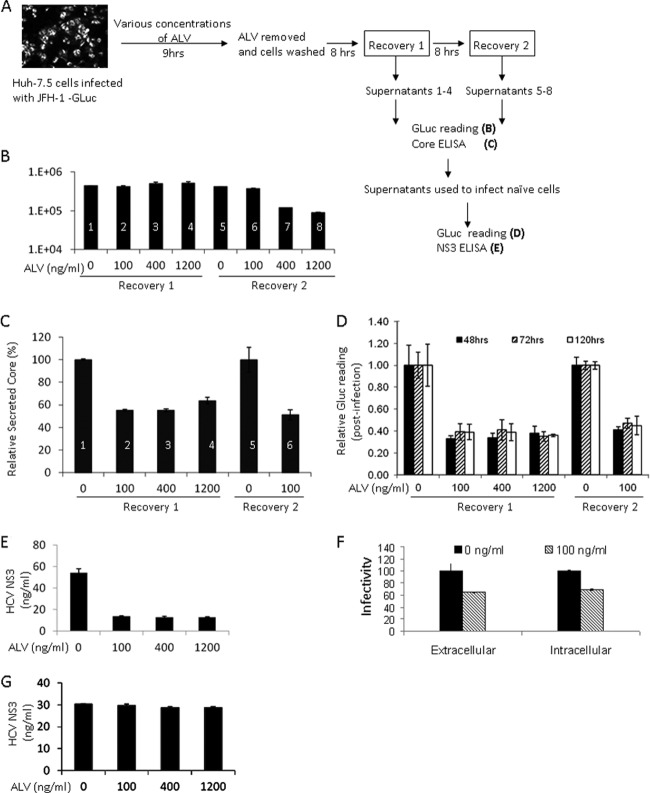
Fig 5.
The CPI ALV inhibits the production of infectious particles in addition to blocking RNA replication. (A) Outline of treatment and recovery conditions for measuring the effect of ALV on HCV assembly. The treatment time was carefully optimized to minimize inhibition of RNA replication. (B) Secreted luciferase activities (GLuc) in the supernatants of treated cells. Samples 1 through 6 showed no reduction with ALV treatment and were used in further analyses of infectivity. (C) Extracellular core levels in the supernatants of treated cells. The untreated sample levels were set to 100%. (D) HCV infectivity in the supernatants of treated cells. The infections were allowed to proceed for 48, 72, or 120 h before infected cell lysates were analyzed for luciferase activity. (E) Intracellular NS3 levels further support the reduction of infectivity in supernatants of treated cells. (F) Similar reductions of HCVcc infectivity intracellularly and in the culture medium upon CPI treatment. Both cell lysate and culture medium of the ALV-treated cells (D and E) were used to infect naïve Huh-7.5 cells, and GLuc activity of the infected cells was determined 5 days later. (G) Lack of carryover ALV in the supernatant. GS5 cells were treated with the same supernatants as in panel E for 120 h, and intracellular NS3 expression is shown.