Fig 2.
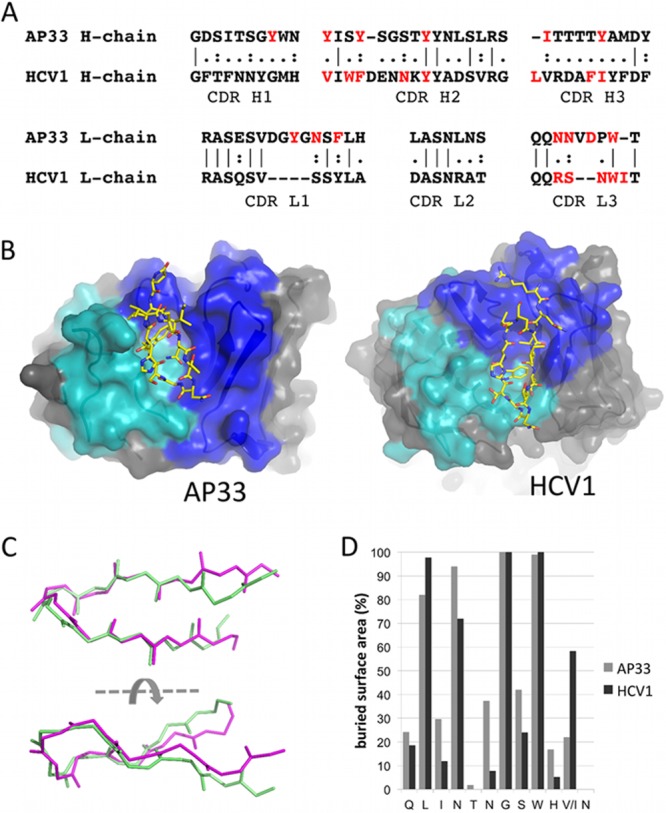
AP33 and HCV1 epitopes adopt a similar conformation. (A) Sequence alignment of AP33 and HCV1 CDRs. The residues of each Fab that interact with the epitope peptide are shown in red. (B) Orientation of the E2 peptide in AP33 (this study) and HCV1 (26) Fab combining sites. Residues of the heavy-chain CDRs are colored blue, and those of the light-chain CDRs are shown in cyan. The peptides are shown as sticks with yellow carbon atoms. (C) Superposition of E2 epitope peptides from complexes with AP33 (pale green) and HCV1 (magenta). Backbone atoms of peptide residues 412 to 422 are shown. (D) Percentage surface area of each peptide residue buried upon binding to AP33 and HCV1, calculated by the software program PISA (28).
