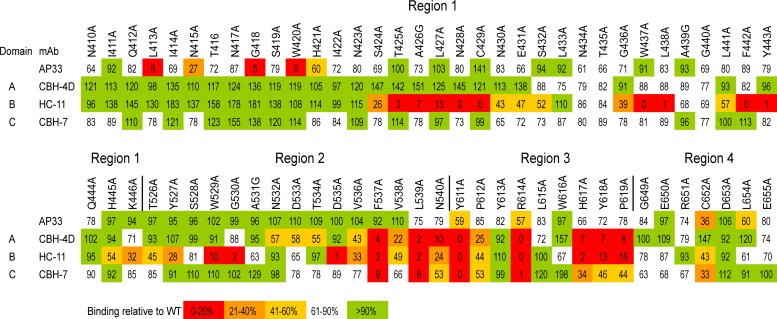
Fig 4.
Epitope mapping of AP33 using alanine-scanning mutagenesis of E2. WT genotype 1a H77c E2 and alanine substitution mutants were expressed in HEK-293T cells. In each mutant, one amino acid residue within four selected regions of E2 (410 to 446, 526 to 540, 611 to 619, and 649 to 655) was replaced by alanine (or glycine, where alanine is the WT residue). The cell lysates were first normalized for E2 on the basis of reactivity to CBH-17, an hMAb to a linear E2 epitope. The normalized lysates were then used in GNA capture ELISA to test the binding of AP33 alongside that of the hMAbs CBH-4D, HC-11, and CBH-7, which recognize nonoverlapping conformational E2 epitopes in antigenic domains A, B, and C, respectively. Antibody binding to each mutant is expressed as a percentage of binding to WT E2. Red indicates 0 to 20%, orange 21 to 40%, yellow 41 to 60%, white 61 to 90%, and green >90% of WT binding.