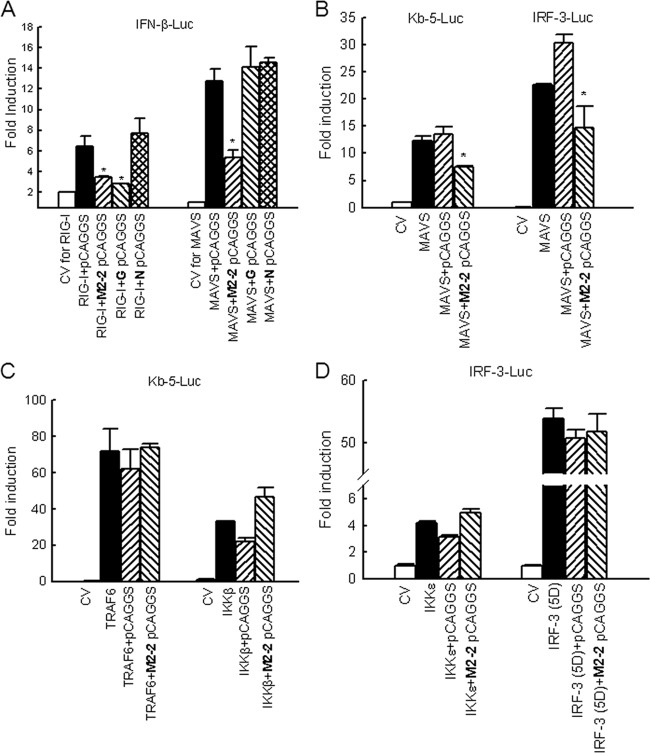
Fig 4.
Inhibition of MAVS-mediated signaling by M2-2 protein. (A) Inhibition of MAVS-induced IFN-β transcription by M2-2. A549 cells in triplicates (24-well plate) were transfected with a luciferase reporter plasmid (IFN-β-Luc), plasmids encoding either RIG-I (0.5 μg/ml) or MAVS (0.2 μg/ml) or their control vectors (CV), or a plasmid expressing hMPV M2-2 or control proteins or the empty vector (0.2 μg/well). Cells were harvested 30 h posttransfection to measure luciferase activity. (B) Inhibition of MAVS-induced NF-κB- or IRF-3-dependent gene transcription by M2-2. HEK 293 cells in triplicates were transfected with a luciferase reporter plasmid (Kb-5-Luc or IRF-3-Luc), plasmids encoding MAVS or its control vector, and a plasmid expressing hMPV M2-2 or the empty vector (0.2 μg/well). Cells were harvested 30 h posttransfection to measure luciferase activity. (C) TRAF6/IKKβ-induced NF-κB-dependent gene transcription was not affected by M2-2. HEK 293 cells in triplicates were transfected with a luciferase reporter plasmid (Kb-5-Luc); plasmids encoding TRAF6, IKKβ, or their control vector; and a plasmid expressing hMPV M2-2 or the empty vector (0.2 μg/well). Cells were harvested 30 h posttransfection to measure luciferase activity. (D) IKKε/IRF-3 (5D)-induced IRF-3-dependent gene transcription was not affected by M2-2. HEK 293 cells in triplicates were transfected with a luciferase reporter plasmid (IRF-3-Luc); plasmids encoding IKKε, the constitutively active form of IRF-3, or their control vector; and a plasmid expressing hMPV M2-2 or the empty vector (0.2 μg/well). Cells were harvested 30 h posttransfection to measure luciferase activity. For all these experiments in panels A to D, luciferase was normalized to the β-galactosidase reporter activity. Data are representative of two to three independent experiments and are expressed as means ± SEs of normalized luciferase activity. *, P < 0.05 relative to signal inducer + pCAGGS group.