Fig 5.
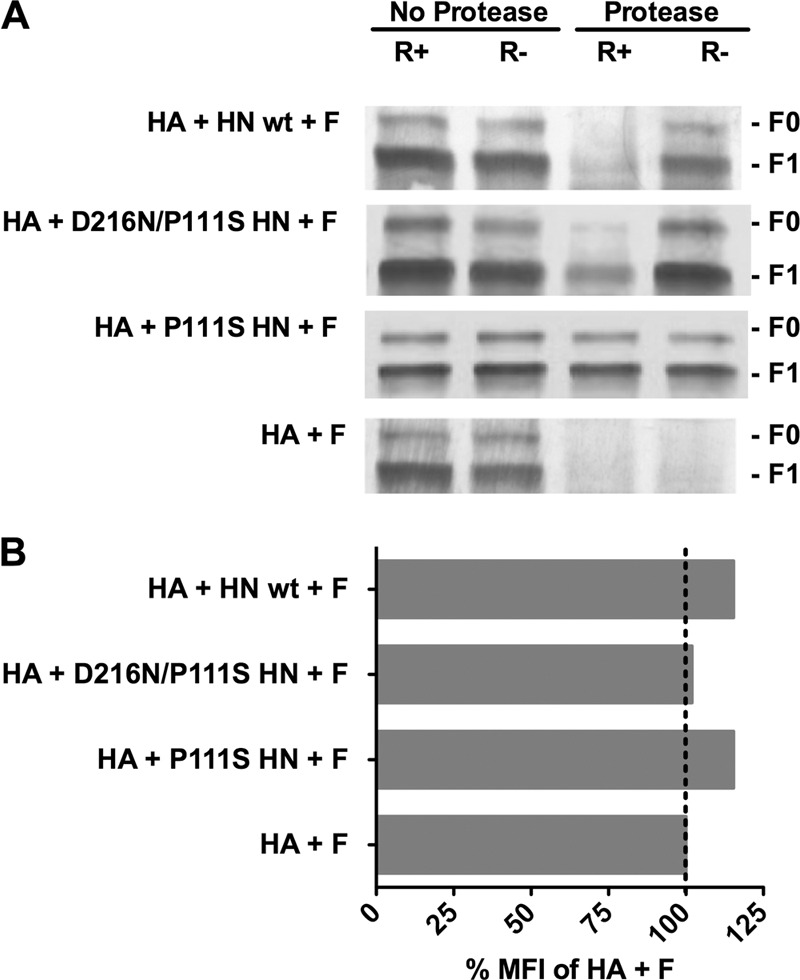
Structural rearrangements in F in are detected by altered protease sensitivity. (A) Monolayers of cells coexpressing F and uncleaved HA with the indicated HN or F and HA alone (bottom row) were incubated for 60 min at 37°C in medium either without zanamivir (receptor-engaged; R+) or with zanamivir to disengage HN from its receptor (R−). After the cells were lysed, the envelope glycoproteins were immunoprecipitated and then incubated in the presence or absence of protease K. The representative Western blot shows F proteolysis, detected by polyclonal anti-F HRC antibodies. F0 (precursor) protein and F1 are indicated. When each HN is present, but not engaging receptor (R−), F does not become protease sensitive. The stalk domain mutant HN (P111S HN) prevents protease digestion of F even when HN is receptor engaged (R+). (B) Flow cytometric analysis of cell surface F expression using rabbit polyclonal anti-F antibodies. The mean fluorescent intensity (MFI) of the cells expressing F with the indicated HN/HA pair is presented as a percentage of the mean fluorescent intensity of cells expressing F with HA alone.
