Abstract
B virus of the family Herpesviridae is endemic to rhesus macaques but results in 80% fatality in untreated humans who are zoonotically infected. Downregulation of major histocompatibility complex (MHC) class I in order to evade CD8+ T-cell activation is characteristic of most herpesviruses. Here we examined the cell surface presence and total protein expression of MHC class I molecules in B virus-infected human foreskin fibroblast cells and macaque kidney epithelial cells in culture, which are representative of foreign and natural host initial target cells of B virus. Our results show <20% downregulation of surface MHC class I molecules in either type of host cells infected with B virus, which is statistically insignificantly different from that observed in uninfected cells. We also examined the surface expression of MHC class Ib molecules, HLA-E and HLA-G, involved in NK cell inhibition. Our results showed significant upregulation of HLA-E and HLA-G in host cells infected with B virus relative to the amounts observed in other herpesvirus-infected cells. These results suggest that B virus-infected cell surfaces maintain normal levels of MHC class Ia molecules, a finding unique among simplex viruses. This is a unique divergence in immune evasion for B virus, which, unlike human simplex viruses, does not inhibit the transport of peptides for loading onto MHC class Ia molecules because B virus ICP47 lacks a transporter-associated protein binding domain. The fact that MHC class Ib molecules were significantly upregulated has additional implications for host-pathogen interactions.
INTRODUCTION
Macacine herpesvirus 1, also known as B virus or herpes B virus and formerly as Cercopithecine herpesvirus 1, belongs to the family Herpesviridae, subfamily, Alphaherpesviridae. B virus is enzootic in all subspecies of macaques (Macaca sp.). In early epidemiological studies, 72% to 92% of wild-caught adult monkeys were found to be seropositive for B virus-reactive antibodies (16, 32, 35, 51, 72, 76). B virus infection in macaques is usually asymptomatic, similar to herpes simplex virus (HSV) infection in humans (33, 34). Zoonotic B virus infection, when not treated in a timely manner, however, leads to encephalitis, encephalomyelitis, and death in up to 80% of those infected (52, 71). With approximately 30,000 macaques imported each year into the United States for biomedical research and a much larger group of captive domestically bred macaques, B virus is a significant occupational hazard for those working with macaques or their cells and tissues. It is also of concern in tourism in Asia, in zoos, and in the growing illegal pet trade. Interestingly, B virus is the only simplex virus that appears to cause zoonotic infections, with 50 known human cases in the United States, and unfortunately, many of these cases have been incompletely documented. Early diagnosis coupled with antiviral intervention using acyclovir or ganciclovir has helped to diminish or prevent virus spread to the central nervous system (CNS) and reduce the death rate to <20%, as reported by the Centers for Disease Control and Prevention (CDC) B Virus Working Group (13). Because of the devastating effects of B virus infection in untreated humans, the inability to cure infections, and the lack of vaccines, it is classified as a biosafety level 4 (BSL-4) agent (12) and is currently designated a select agent by the U.S. Department of Homeland Security.
B virus infection of nonmacaque monkeys is rapidly fatal (14, 21, 30, 44, 73) and is observed mostly when macaques have been cohoused with other monkey species. Virus can be transmitted from infected animals, infected tissues (4, 5, 15, 26, 27), or contaminated surfaces or, in one case, through human-to-human contact (24). Because B virus is fatal in foreign hosts such as humans, it is important to understand the pathogenesis of this zoonotic infection in order to develop effective prevention and control strategies. Interestingly, B virus-infected macaques generally show a strong, high-titer IgG response within 2 to 4 weeks of infection, although not always. In humans, however, B virus infection generally induces only low levels of IgG, if any at all, following antiviral intervention. Survivors of B virus infection show detectable levels of B virus-specific IgG eventually, unless they remain on lifelong antiviral therapy. B virus-specific antibodies persist for decades in untreated survivors, fluctuating significantly, suggesting periodic reactivation of latent B virus. Morbidity due to reactivation has been documented on two occasions (17).
In the cases of zoonotic B virus infection, host restriction of virus spread appears largely unsuccessful and timely antiviral therapy is critical for survival. Considering the cells that are critical to the establishment of effective innate responses, we examined mechanisms by which B virus differed from its close relatives HSV-1 and HSV-2 by identifying differences in selected host-mounted innate defenses.
Most known herpesviruses downregulate major histocompatibility complex (MHC) class Ia surface expression. HSV-1 and HSV-2 downregulate surface MHC class Ia by blocking host TAP (transporter-associated protein) with viral ICP47, resulting in the sequestration of MHC class I in the endoplasmic reticulum, preventing HSV-specific CD8+ T-cell recognition of infected cells (18, 25, 59, 74). This downregulation, however, leads to susceptibility to NK cell cytotoxicity (29). NK cell cytotoxicity of infected cells during HSV infection limits the spread of the virus, resulting in a relative balance between virus spread and the host immune response. In this study, we explore the regulation of MHC class I expression on the infected cell surface to determine the possibility of susceptibility of B virus-infected cells to NK cell-mediated cell death. The protein responsible for MHC class I downregulation in HSV-1-infected cells is immediate-early protein ICP47, which is also present in B virus but lacking the TAP binding domain present in ICP47 of HSV-1 and HSV-2, as we first demonstrated (56). The TAP binding domain in B virus ICP47 led us to hypothesize that B virus regulation of MHC class I is unlike that of HSV, presumably protecting infected cells from NK cell cytotoxicity, buying the virus time to replicate in the protected environment of the cell unrecognized by NK cells.
The polymorphic MHC class I molecules include HLA-A, -B, and -C and are categorized as classical MHC class I or MHC class Ia molecules. The less polymorphic MHC class I molecules, HLA-E, HLA-F, HLA-G, and HLA-H, are called nonclassical MHC class I or Ib molecules. MHC class Ia and Ib molecules are both recognized by natural killer cells, and these have an inhibitory effect on NK cell function. The presence of surface HLA-E and HLA-G predominantly inhibits NK cell cytotoxic function. HLA-E binds to the CD94/NKG2A inhibitory receptor complex (7, 40), while HLA-G binds to inhibitory receptors, leukocyte Ig-like receptor 1 (22), and the NK cell killer inhibitory receptor (KIR2DL4) (61). HLA-G, in addition to inhibiting NK cells (55, 63, 64), inhibits CD8+ T cells (41, 65) by binding to ILT-2 and ILT-4 inhibitory receptors (42, 67).
MATERIALS AND METHODS
Cells and viruses.
Human foreskin fibroblast (HFF) cells (ATCC CRL-2097) obtained from ATCC (Manassas, VA) were maintained as monolayers in minimal essential medium (MEM), which was purchased from Mediatech, Inc. (Manassas, VA), and supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), 1% nonessential amino acids, and 1% sodium pyruvate. Cells between passages 6 and 13 were used for all experiments with HFF cells. Macaque kidney epithelial cells (MK2; ATTC CCL-7) were obtained from ATCC and maintained as a monolayer in Dulbecco's modified Eagle's medium (Mediatech, Inc.) with 10% FBS. B virus lab strain E2490, passages 71 to 73, originally obtained from E. Hull; HSV-1 strain MacIntyre (lot VR-539), purchased from ATCC; and herpesvirus papio 2 (HVP-2) lab strain X2980, from the Southwest Foundation for Biomedical Research, San Antonio, TX (isolated by J.H.), were used in the experiments presented here. All virus stocks were prepared using Vero cells (lot CCL-81) obtained from ATCC. All experiments with B virus were done in a CDC-certified BSL-4 laboratory in compliance with reference 12 and the U.S. Department of Justice homeland security directives on select agents. Georgia State University is a registered select agent facility in good standing.
Plaque assays.
Virus infectivity in different cell lines was determined by using standard plaque assays to establish the efficiency of plating and the quantity of virus needed for infection to achieve equivalent results with each cell line. For the plaque assays, Vero or HFF cells were grown to subconfluence in six-well plates. The medium was removed, and each virus suspension, serially diluted (1:10 to a final dilution of 1:1010), was added to duplicate wells. Virus was adsorbed in 400 μl for 1 h at 37°C in a 5% CO2 incubator. At 1 h postinfection (hpi), the medium was removed and fresh MEM containing 2% fetal calf serum mixed with 1% methylcellulose was added to each well. The plates were incubated for 48 hpi, after which the medium overlay was removed and the wells were washed twice with PBS (phosphate-buffered saline) and fixed with 100% methanol. Fixed cells were stained with 0.2% crystal violet, and the plaques were counted.
Western blot assays.
HFF cells were infected with B virus or control HSV-1 at a multiplicity of infection (MOI) of 10. Prepared infected cell lysates were resuspended in Laemmli sample buffer containing 5% 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO). The solution was boiled for 3 min and then fractionated by 10% SDS-PAGE. Subsequently, proteins were transferred onto nitrocellulose membrane (0.45 μm; GE Healthcare, Piscataway, NJ) by electroblotting for 2 h at 65 V. Transferred proteins were probed with mouse anti-human HLA-A, -B, and -C clone W6/32 antibody (Sigma-Aldrich, St. Louis, MO) at a predetermined dilution of 1:1,000. Mouse anti-human polyclonal HLA-E (Abcam, Cambridge, MA), at a concentration of 3 μg/ml, was used to determine the total HLA-E expression in HFF cells. Mouse anti-human HLA-G clone MEM G/1 (10 μg/ml; Abcam) was used to measure the total HLA-G protein. Rabbit anti-human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:10,000; purchased from Abcam) was used to detect GAPDH expression to evaluate the consistency of the loading volume of individual wells. Goat anti-mouse antibody conjugated to horseradish peroxidase was used (1:20,000) to detect the mouse anti-human and rabbit anti-human primary antibodies. Respective bands were detected by using ECL plus Western blotting detection reagent (GE Healthcare), and band intensity analysis was performed by using ImageJ (NIH) imaging software.
Flow cytometry.
HFF and MK2 cells were infected at subconfluency (MOI of 10) or left uninfected. At 18 hpi, cells were washed with PBS. Cells were trypsinized and fixed with 4% formaldehyde at room temperature for 1 h. Fixed cells were washed three times with 1% bovine serum albumin (BSA) in PBS. For total antigen, formaldehyde-fixed cells were treated with 0.1% Triton X to permeabilize them and then labeled with fluorescein isothiocyanate (FITC)-conjugated mouse monoclonal anti-human HLA class I clone W6/32 (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions (10 μl/1 × 106 cells), phycoerythrin (PE)-conjugated mouse monoclonal anti-human HLA-E clone 3D12 (20 μl/1 × 106 cells), or anti-human HLA-G clone 87G (20 μl/1 × 106 cells) (BioLegend, San Diego, CA). Subsequently, cells were incubated at room temperature for 1 h in the dark. To determine if all of the HFF cells were infected with B virus, uninfected and B virus-infected HFF cells were incubated with rhesus anti-B-virus serum (1:20 dilution) for 1 h. Subsequently, cells were washed with fluorescence-activated cell sorter (FACS) buffer (1% BSA in PBS) and labeled with PE-conjugated anti-human IgG (BioLegend, San Diego, CA), 5 μl/1 × 106 cells, for 20 min in the dark. Labeled cells were washed twice in cold PBS and then analyzed using BD FACS Canto (BD Biosciences, San Jose, CA). MHC class I protein expression was measured in terms of the mean fluorescence intensity (MFI) emitted by labeled cells. Uninfected, unlabeled cells were used as a negative control to assess autofluorescence. The MFI of labeled cells was normalized by subtracting the MFI of unlabeled cells from the MFI of labeled cells. The MFI ratio was calculate by using the following formula: MFI ratio = MFI of infected cells/MFI of uninfected cells.
Statistical analysis.
Data collection by flow cytometry analysis for surface expression of MHC class I molecules was repeated three times. The paired one-tailed Student t test was used to calculate P values. If the P value was ≤0.05, the two groups were found to be significantly different from each other.
RESULTS
Total antigen and surface expression of MHC class Ia in B virus-infected HFF cells.
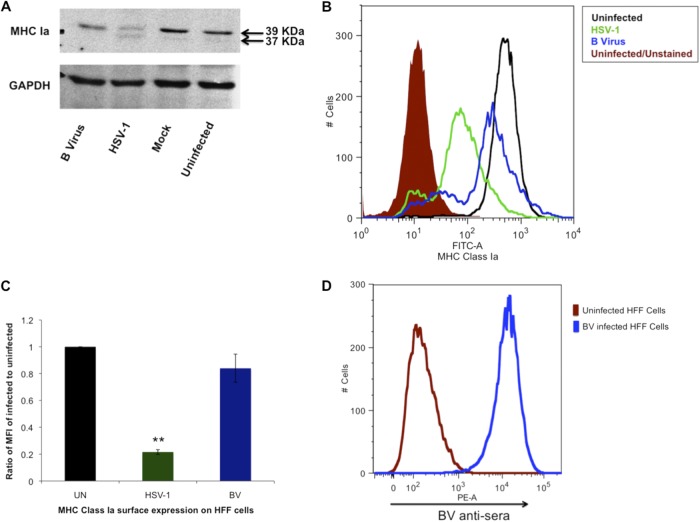
B virus-infected HFF and Vero cells were compared to establish whether virus replication efficiency differed between the two cell lines. Vero cells were approximately three times more susceptible to B virus than HFF cells, as measured by plaque assay. No difference in the efficiency of plating of HSV-1 was observed between the two cell lines. Once efficiency of plating was determined for each cell line, experiments were performed by using the adjusted amounts of each virus for each cell line to evaluate the effect of each virus in each cell system with respect to MHC class I expression. The HFF cells were infected with B virus or HSV-1 at an MOI of 10 for 18 h at 37°C. Control cells were either left uninfected or mock infected by adsorption with uninfected cells prepared as described for virus-infected cell lysates. Following fractionation and Western blotting, the presence of MHC class Ia (HLA-A, -B, and -C) molecules in these samples was measured. (Fig. 1A). The band intensity at 39 kDa representing the glycosylated and functional form of MHC class Ia was observed in B virus-infected cells. This was comparable to levels in the controls, indicating no significant differences in the expression of MHC class Ia, unlike with HSV-1-infected cells, where we observed two bands, one at 39 kDa and another at 37 kDa with significantly lower band intensities than the controls, as shown previously by York et al. in 1994 (75). The lower-molecular-mass band corresponds to nonglycosylated MHC class Ia (sequestered MHC class Ia) (75). These results suggested that B virus-infected cells have normal levels of surface expression of HLA-A, -B, and -C, and in order to confirm these results, we measured the surface expression of HLA-A, -B, and -C on B virus-infected, HSV-1-infected, and uninfected cells at 18 hpi by using flow cytometry. Flow cytometry results showed an only 14% reduction in MHC class Ia surface expression in B virus-infected cells compared to that in uninfected cells. This downregulation was found to be insignificant as determined by Student t test, where the P value was >0.05 (Fig. 1B and C). A significant difference in the surface expression of MHC class Ia was observed, however, between uninfected and HSV-1-infected cells (Fig. 1B and C), where HSV-1-infected cells showed a 78% reduction in surface MHC class Ia expression compared to that on uninfected cells (Fig. 1B and C) over the course of multiple repetitions. Significant differences in surface MHC class I expression were also observed between B virus-infected and HSV-1-infected cells. Western blot analyses, along with flow cytometry data, showed no significant reduction in MHC class Ia in B virus-infected cells. To show that all of the cells were indeed infected with B virus, uninfected and B virus-infected HFF cells were incubated with anti-B-virus serum and labeled with PE-conjugated anti-human IgG. Our results suggest that there was approximately 98% infection (Fig. 1D).
Fig 1.
MHC class Ia regulation in B virus-infected HFF cells. (A) HFF cells were infected for 18 h with B virus or HSV-1 at an MOI of 10, and the amounts of total MHC class Ia (HLA-A, -B, and -C) antigen in the cell lysates were quantified by using anti-human MHC class I clone W6/32. The band corresponding to a 39-kDa molecular mass represents normal MHC class Ia, while the 37-KDa band represents nonglycosylated MHC class Ia due to incomplete processing of MHC class Ia. (B) Uninfected HFF cells and HSV-1- or B virus-infected (MOI, 10) HFF cells were formaldehyde fixed and stained for detection of surface MHC class Ia by using FITC-conjugated anti-human MHC class Ia W6/32 antibody (FITC-A). The shaded histogram represents uninfected cells unstained for autofluorescence, the black histogram represents uninfected stained cells, the blue histogram represents B virus-infected stained cells, and the green histogram represents HSV-1-infected stained cells. (C) MFIs of B virus (BV)- and HSV-1-infected HFF cells relative to the MFI of uninfected (UN) HFF cells. **, P ≤ 0.01. (D) The blue histogram represents B virus-infected HFF cells fixed, labeled with BV antiserum, and stained with anti-human IgG (PE-A)-conjugated antibody.
Regulation of MHC class Ia molecules is independent of the host cell species.
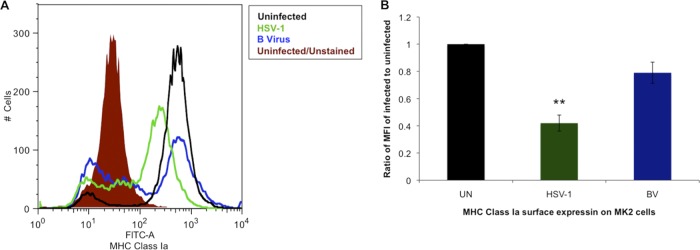
Our data analyses revealed that B virus does not efficiently downregulate MHC class Ia cell surface expression in HFF cells compared to that on HSV-1-infected HFF cells. To determine if this observation was consistent in macaque-derived epithelial cells (MK2), cells from the natural host species of B virus, we infected MK2 cells (MOI, 10) as described in Materials and Methods. The results of our experiments showed that B virus did not downregulate MHC class Ia surface expression as efficiently as HSV-1 does in macaque host cells. B virus-infected cells showed a ≤21% (P value, >0.05) reduction of surface MHC class Ia expression compared to that on uninfected cells, while HSV-1-infected cells showed 58% downregulation (P values, <0.05) compared to the level on uninfected cells (Fig. 2A and B). These data suggest that B virus does not significantly reduce normal levels of surface MHC class Ia molecule expression on macaque cells, nor does it interfere with this process significantly in cells derived from human hosts.
Fig 2.
MHC class Ia is not downregulated in B virus-infected macaque epithelial cells. (A) MK2 cells were infected with B virus or HSV-1 at an MOI of 10, and formaldehyde-fixed cells were stained for surface MHC class Ia using FITC-conjugated anti-human MHC class I clone W6/32 (FITC-A). The shaded histogram represents uninfected cells unstained for autofluorescence, the black histogram represents uninfected stained cells, the blue histogram represents B virus-infected stained cells, and the green histogram represents HSV-1-infected stained cells. (B) MFIs of B virus (BV)- and HSV-1-infected MK2 cells relative to the MFI of uninfected (UN) HFF cells. **, P ≤ 0.01.
Regulation of HLA-E surface expression on B virus-infected cells.
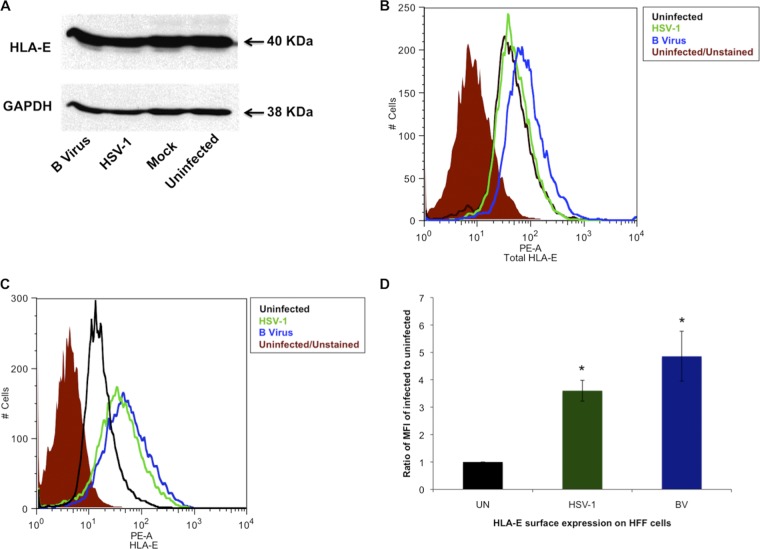
HLA-E belongs to MHC class Ib molecules, and it binds to β-microglobulin. HLA-E presentation is known to be both TAP dependent (9, 39) and TAP independent (20, 43, 69). HLA-E molecules present the leader peptide of MHC class Ia molecules, and these, in turn, bind to inhibitory receptors on NK cells (40). To further explore this, we analyzed B virus- and HSV-1-infected cells for total and surface expression of HLA-E. The total expression of HLA-E was quantified by using Western blot assays of B virus- or HSV-1-infected cells that were SDS-PAGE fractionated and subsequently reacted with polyclonal anti-human HLA-E antibody. Our results showed no HLA-E expression level differences between the virus-infected cells (Fig. 3A). To verify that there was, indeed, no significant difference in total HLA-E expression between B virus-infected and uninfected cells, we used a PE-conjugated mouse anti-HLA-E monoclonal antibody (3D12) and determined the total HLA-E antigen expression by using flow cytometry. Our results again showed that there was no difference between uninfected HFF cells and B virus- or HSV-1-infected HFF cells. There was an only 1.7-fold difference in total HLA-E expression between B virus-infected and uninfected cells (Fig. 3B). We then proceeded to quantify HLA-E surface expression by using flow cytometry. Formaldehyde-fixed infected and uninfected cells were labeled with PE-conjugated mouse anti-human HLA-E (clone 3D12), which detects only cell surface HLA-E molecules. Because HLA-E cell surface expression is both TAP dependent and TAP independent, we expected B virus-infected cells to express relatively more cell surface HLA-E than HSV-1-infected cells do. Data analysis revealed greater cell surface HLA-E expression on both B virus (4.86-fold)- and HSV-1 (3.6-fold)-infected cells than on uninfected cells, with no significant difference between B virus- and HSV-1-infected cells (Fig. 3C and D). These data show, for the first time, the presence of HLA-E on B virus-infected cells, as well as on HSV-1-infected cells.
Fig 3.
Regulation of HLA-E in HFF cells infected with B virus. (A) Western blot analysis of HFF cells infected with B virus or HSV-1 and probed with anti-HLA-E polyclonal antibody. (B) HFF cells were infected with B virus or HSV-1 at an MOI of 10, and total HLA-E expression was determined by using flow cytometry. The shaded histogram represents uninfected cells unstained for autofluorescence, the black histogram represents uninfected cells stained with PE-conjugated anti-HLA-E antibody clone 3D12 (PE-A), the blue histogram represents B virus-infected stained cells, and the green histogram represents HSV-1-infected stained cells. (C) HLA-E surface expression determined by using flow cytometry. (D) MFIs of B virus (BV)- and HSV-1-infected HFF cells relative to the MFI of uninfected (UN) HFF cells. *, P ≤ 05.
HLA-G is differentially regulated on B virus-infected cell surfaces.
The presence of HLA-G on an infected cell surface can inhibit NK cell recognition by binding to KIR2DL4 receptors on NK cells (61). HLA-G also binds to the ILT4 inhibitory receptor on T cells (67), thus inhibiting T-cell activation. Western blot analysis was performed to identify all forms of HLA-G expression present, with bands ranging from 39 kDa to 21 kDa. We observed no differences in total HLA-G levels between B virus-infected and uninfected HFF cells. In contrast, HSV-1-infected cells showed the expression of different isoforms (Fig. 4A). Therefore, we determined the expression of functional HLA-G isoforms, membrane-bound HLA-G1 and soluble HLA-G5, which are involved in inhibiting NK and CD8+ T-cell cytotoxicity. Analysis of surface expression of HLA-G1 and HLA-G5 was performed using formaldehyde-fixed B virus-infected, HSV-1-infected, and uninfected HFF cells reacted with PE-conjugated anti-HLA-G antibody (clone 87G). For total expression, formaldehyde-fixed cells were treated with 0.1% Triton X and then reacted with PE-conjugated anti-HLA-G antibody (clone 87G). HSV-1-infected cells showed 3.0-fold more surface HLA-G than uninfected cells (Fig. 4B and C); however, there was an only 1.6-fold increase when both the membrane-bound (HLA-G1) and soluble (HLA-G5) forms were measured (Fig. 4D). B virus-infected cells, on the other hand, were associated with significantly higher (7.7-fold) membrane-bound HLA-G1 expression than uninfected cells (Fig. 4B and C). When both the membrane-bound and soluble forms (HLA-G1 and HLA-G5, respectively) on B virus-infected and uninfected cells were compared, there was a 12.5-fold increase (Fig. 4B), indicating that upregulation of HLA-G1 and HLA-G5 is induced in B virus-infected HFF cells.
Fig 4.
Differential regulation of HLA-G in HFF cells infected with B virus. (A) Western blot analysis of HFF cells infected with B virus or HSV-1 using anti-HLA-G antibody (clone MEM G/1) to detect all forms of HLA-G. (B) HFF cells were infected with B virus or HSV-1 at an MOI of 10, and surface HLA-G1 and -expression was determined by using flow cytometry. The shaded histogram represents uninfected cells unstained for autofluorescence, the black histogram represents uninfected cells stained with PE-conjugated anti-HLA-G clone 87G (PE-A), the blue histogram represents B virus-infected stained cells, and the green histogram represents HSV-1-infected stained cells. (C) MFIs of B virus (BV)- and HSV-1-infected HFF cells relative to that of uninfected (UN) HFF cells. *, P ≤ 02; **, P ≤ 0.005. (D) HLA-G total expression determined by using flow cytometry.
DISCUSSION
In order for a virus to successfully propagate in a host without causing life-threatening adverse effects, there must be a fine balance between the rate of virus spread and the immune responses induced within the host against a specific virus. Herpesviruses remain in the host, establishing life-long latent infections that can reactivate unpredictably. Failure to maintain a balance between the host and pathogen can lead to a dead-end host for the virus or unproductive virus replication, neither of which is favorable to the virus. When a host fails to induce a robust defense against a virus, the virus infection overcomes the host, resulting in morbidity and even death, a common outcome of zoonotic infections like B virus, Marburg virus, Ebola virus, and rabies virus infections, to name several. In the experiments presented here, we hypothesized that B virus infection dysregulates innate defenses in host target cells differently from HSV, which has coevolved with human hosts over millions of years and as a result, the host and pathogen coexist successfully. B virus, not having the benefit of coevolution with human hosts, causes death in up to 80% of untreated, zoonotically infected individuals. Data presented here establish that infections with B virus, which lacks a functional TAP domain in ICP47 homologs, have outcomes strikingly different, with respect to MHC class I cell surface expression, from those with other simplex viruses. The classical way in which investigators have long thought about MHC class I downregulation during herpesvirus infections is not supported in the cases of B virus-infected cells presented here. We also investigated the expression of MHC class I expression in HVP-2-infected HFF and Vero cells. HVP-2-infected HFF and Vero cells show almost 30% MHC class I surface downregulation (see Fig. S1A, B, and C in the supplemental material). Both HFF and Vero cells are foreign cell lines and are not naturally infected with HVP-2. We have not yet identified MHC class I surface expression in an HVP-2-infected primary baboon cell line to determine the extent of MHC class I regulation in a natural host cell line.
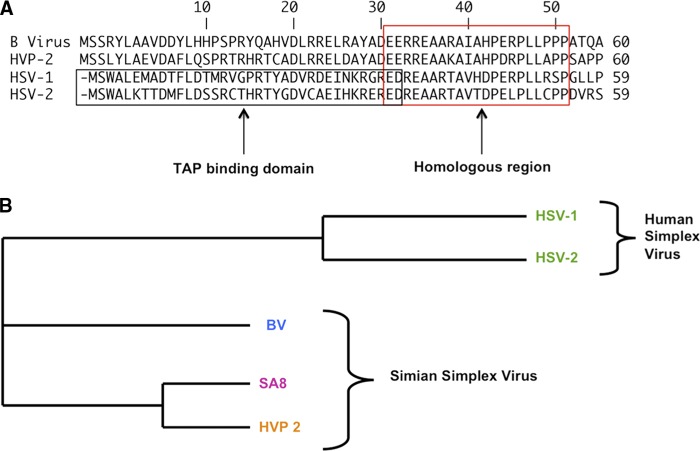
MHC class I expression is an important aspect of the immune system bridging innate and adaptive immune responses. For this reason, many persistent viruses downregulate functional MHC class I presentation on infected cell surfaces. This is true especially in the case of herpesviruses, where previously studied herpesviruses downregulate MHC class I. The critical 3- to 35-amino-acid sequence of ICP47 of simplex viruses, which binds to the peptide-binding region of TAP, inhibiting peptide loading, is conserved in both HSV-1 and HSV-2 (23, 49, 57, 66, 68). Other alphaherpesviruses, such as varicella-zoster virus, pseudorabies virus, equine herpesvirus 1, and bovine herpesvirus 1, retain the ability to downregulate MHC class I (1, 2, 3, 37, 38, 62, 74) Cytomegalovirus, a betaherpesvirus, also encodes more than one protein to inhibit the surface expression of MHC class I (53). Nonhuman primate simplex viruses such as B virus, HVP-2, and simian herpesvirus (SA8) express the US12 gene encoding ICP47; however, the conserved sequence of the TAP binding domain of HSV-1/2 ICP47 is absent (6). Because of the differences between the ICP47 homologues of B virus, HVP-2, and the human simplex viruses (Fig. 5), viz., the absence of the TAP binding domain, we speculated that the levels of cell surface expression of MHC class I in B virus-infected cells should be relatively unaffected, unlike what is observed during HSV-1 infection. Given the observations in experiments reported in this paper and the suggestions of Bigger and Martin (6), we speculate that ICP47 of nonhuman primate simplex viruses may have other functions in the absence of TAP binding domains. Thus, on the basis of our experiments, we suggest that nonhuman primate simplex viruses lacking a TAP binding domain do not utilize downregulation of cell surface expression of MHC class I.
Fig 5.
ICP47 sequence comparison between human herpesviruses (HSV-1 and HSV-2) and simian herpesviruses (B virus, HVP-2, and SA8). (A) Sequence homology among HSV-1, HSV-2, B virus, and HVP-2 ICP47 in amino acids 1 to 60. The N-terminal region of HSV ICP47 is the TAP binding domain, which is not conserved in B virus ICP47 or in HVP-2 ICP47. The C-terminal region of ICP47, however, is the most homologous region in all four viruses. (B) Phylogenic tree indicating the divergence of ICP47 between human and simian HSVs. This phylogenic tree was generated using ClustalW program. BV, B virus.
Since NK cells recognize the MHC class I molecules presented on normal cells to distinguish between “self” and “nonself” or infected and uninfected cells, the absence of MHC class I molecules from the cell surface triggers NK cells to kill target cells unless NK cell-inhibitory ligands are expressed on the target cells as well. HSV-1-infected cells are sensitive to accessory cell-dependent (28) and -independent NK cell lysis (29), and NK cells play a direct role in reducing HSV-1 replication in vivo (60). HSV-1-infected patients who lack NK cells, in fact, fail to limit the infection and suffer from severe herpesvirus infection (45, 50). NK cells require activation ligands on target cells or accessory cells to bind to the receptors necessary for NK cell activation. Ultimately, a balance must exist between activation and inhibitory ligands in order to modulate NK cell activity. Because B virus does not block MHC class I expression on infected cell surfaces, we suggest here that B virus-infected cells do not trigger NK cell defenses, and this is likely one reason why humans fail to restrict infection. This, however, does not explain why in macaques disease is mild to asymptomatic in the absence of NK-mediated killing of infected cells.
To understand how B virus-infected cells may escape NK cell lysis, we also evaluated HLA-E and HLA-G MHC class Ib molecules. MHC class Ib proteins are less polymorphic than MHC class Ia proteins. HLA-E associates with β2-microglobulin and is expressed on the cell surface. These molecules act as ligands presenting MHC class Ia leader peptide to CD94/NKG2A, an inhibitory receptor of NK cells. Surface expression of HLA-E was also observed in TAP1-deficient individuals (20). Our results show unequivocally that HLA-E expression during HSV-1 infection is TAP independent because TAP is blocked by ICP47; however, upregulation of HLA-E was clearly observed in our experiments. Whether HLA-E serves to downregulate the NK killing reported for HSV-1-infected cells that downregulate MHC class Ia molecules on infected cell surfaces remains to be investigated.
HLA-G is an MHC class Ib molecule that binds to the killer cell immunoglobulin-like receptor KIR2DL4 inhibitory receptor of NK cells. These molecules are expressed mostly on placental cells (specifically, cytotrophoblast cells) to protect the fetus from maternal NK cell-induced cytolysis. HLA-G is the least polymorphic of the MHC class I molecules, consisting of seven isoforms resulting from alternative splicing (19, 31, 36, 48, 54) and is differentially expressed in different cells (10, 11, 46, 70). The membrane-bound isoforms HLA-G1, HLA-G2, HLA-G3, and HLA-G4 are involved in inhibiting NK cell and cytotoxic T lymphocyte lysis (63, 65). In our experiments, we observed surface expression on HLA-G-infected fibroblasts, with significantly higher expression on B virus-infected cells than on HSV-1-infected cells. HLA-G, especially soluble HLA-G5 and membrane-bound HLA-G1 excreted by proteolytic cleavage, is implicated in many immunosuppressive mechanisms, e.g., impairment of inflammatory cytokines; modulation of T cells, dendritic cells, and macrophages (58); and inhibition of T-cell chemotaxis (47), besides inhibition of NK and CD8+ T-cell activation. Because of the observed upregulation of HLA-G1 and G5 in B virus-infected cells and because of the various immunomodulatory mechanisms employed by HLA-G, we suggest that the presence of HLA-G may be one reason for the inefficient immune response noted by our laboratory during zoonotic B virus infections (unpublished data). The presence of soluble HLA-G in the serum of B virus-infected humans and macaques remains to be evaluated. Interestingly, the macaque ortholog of human HLA-G, Mamu-G, is encoded by a pseudogene; however, a protein encoded by a functionally similar gene, Mamu-AG, which is more closely related to HLA-A, was found in macaques (8). Anti-human HLA-G antibodies do not cross-react with Mamu-AG, and because of the unavailability of commercial Mamu-AG antibodies, Mamu-AG levels in B virus-infected macaque cell lines were not determined in the present study.
Downregulation of MHC class Ia molecules leads to NK cell cytotoxicity. B virus-infected cells, unlike HSV-1-infected cells, do not exhibit significant downregulation of MHC class I. Our data suggest that B virus subverts NK cell defenses specifically by not interfering with MHC class Ia expression on the cell surface and additionally by upregulating inhibitory MHC class Ib cell surface molecules to avoid NK cell lysis. Evasion of NK killing is probably one of a number of important factors that enable B virus to successfully invade the CNS during zoonotic B virus infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by NIH P40 RR05162-20 and was made possible by funding from the Georgia Research Alliance to provide for the specialized BSL-4 facilities required for studies of B virus. We are also grateful to the Molecular Basis of Disease Program resources available to M.V. during dissertation studies when much of this work was performed.
Footnotes
Published ahead of print 12 September 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Abendroth A, Lin I, Slobedman B, Ploegh H, Arvin AM. 2001. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J. Virol. 75:4878–4888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ambagala AP, Gopinath RS, Srikumaran S. 2004. Peptide transport activity of the transporter associated with antigen processing (TAP) is inhibited by an early protein of equine herpesvirus-1. J. Gen. Virol. 85:349–353 [DOI] [PubMed] [Google Scholar]
- 3. Ambagala AP, Hinkley S, Srikumaran S. 2000. An early pseudorabies virus protein down-regulates porcine MHC class I expression by inhibition of transporter associated with antigen processing (TAP). J. Immunol. 164:93–99 [DOI] [PubMed] [Google Scholar]
- 4. Artenstein AW, Hicks CB, Goodwin BS, Jr, Hilliard JK. 1991. Human infection with B virus following a needlestick injury. Rev. Infect. Dis. 13:288–291 [DOI] [PubMed] [Google Scholar]
- 5. Benson PM, Malane SL, Banks R, Hicks CB, Hilliard JK. 1989. B virus (Herpesvirus simiae) and human infection. Arch. Dermatol. 125:1247–1248 [PubMed] [Google Scholar]
- 6. Bigger JE, Martin DW. 2004. Identification of an ICP47 homologue in simian agent 8 (SA8). Virus Genes 28:223–225 [DOI] [PubMed] [Google Scholar]
- 7. Borrego F, Ulbrecht M, Weiss EH, Coligan JE, Brooks AG. 1998. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence-derived peptides by CD94/NKG2 confers protection from natural killer cell-mediated lysis. J. Exp. Med. 187:813–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boyson JE, Iwanaga KK, Golos TG, Watkins DI. 1997. Identification of a novel MHC class gene, Mamu-AG, expressed in the placenta of a primate with an inactivated G locus. J. Immunol. 159:3311–3321 [PubMed] [Google Scholar]
- 9. Braud VM, Allan DS, Wilson D, McMichael AJ. 1998. TAP- and tapasin-dependent HLA-E surface expression correlates with the binding of an MHC class I leader peptide. Curr. Biol. 8:1–10 [DOI] [PubMed] [Google Scholar]
- 10. Carosella ED, Dausset J, Kirszenbaum M. 1996. HLA-G revisited. Immunol. Today 17:407–409 [DOI] [PubMed] [Google Scholar]
- 11. Carosella ED, Paul P, Moreau P, Rouas-Freiss N. 2000. HLA-G and HLA-E: fundamental and pathophysiological aspects. Immunol. Today 21:532–534 [PubMed] [Google Scholar]
- 12. Chosewood LC, Wilson DE. (ed). 2009. Biosafety in microbiological and biomedical laboratories manual. Centers for Disease Control and Prevention, Atlanta, GA [Google Scholar]
- 13. Cohen JI, et al. 2002. Recommendations for prevention of and therapy for exposure to B virus (cercopithecine herpesvirus 1). Clin. Infect. Dis. 35:1191–1203 [DOI] [PubMed] [Google Scholar]
- 14. Coulibaly C, et al. 2004. A natural asymptomatic herpes B virus infection in a colony of laboratory brown capuchin monkeys (Cebus apella). Lab. Anim. 38:432–438 [DOI] [PubMed] [Google Scholar]
- 15. Davenport DS, et al. 1989. B virus infections in humans—Michigan. MMWR Morb. Mortal. Wkly. Rep. 38(26):453–454 [PubMed] [Google Scholar]
- 16. Engel GA, et al. 2002. Human exposure to herpesvirus B-seropositive macaques, Bali, Indonesia. Emerg. Infect. Dis. 8:789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fierer J, Bazeley P, Barude AI. 1973. Herpes B virus encephalomyelitis presenting as ophthalmic zoster: a possible latent infection reactivated. Ann. Intern. Med. 79:225–228 [DOI] [PubMed] [Google Scholar]
- 18. Früh K, et al. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415–418 [DOI] [PubMed] [Google Scholar]
- 19. Fujii T, Ishitani A, Geraghty DE. 1994. A soluble form of the HLA-G antigen is encoded by a messenger ribonucleic acid containing intron 4. J. Immunol. 153:5516–5524 [PubMed] [Google Scholar]
- 20. Furukawa H, et al. 1999. Cell surface expression of HLA-E molecules on PBMC from a TAP1-deficient patient. Tissue Antigens 53:292–295 [DOI] [PubMed] [Google Scholar]
- 21. Gay FP, Holden M. 1933. The herpes encephalitis problem, II. J. Infect. Dis. 53:287–303 [Google Scholar]
- 22. Gonen-Gross T, et al. 2003. Complexes of HLA-G protein on the cell surface are important for leukocyte Ig-like receptor-1 function. J. Immunol. 171:1343–1351 [DOI] [PubMed] [Google Scholar]
- 23. Gorbulev S, Abele R, Tampe R. 2001. Allosteric crosstalk between peptide-binding, transport, and ATP hydrolysis of the ABC transporter TAP. Proc. Natl. Acad. Sci. U. S. A. 98:3732–3737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Griffin DG, et al. 1987. B virus infection in humans—Pensacola, Florida. MMWR Morb. Mortal. Wkly. Rep. 36(19):289–290, 295–296 [PubMed] [Google Scholar]
- 25. Hill A, et al. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411–415 [DOI] [PubMed] [Google Scholar]
- 26. Hilliard J, Scinicariello F. 1992. B virus transmission from monkey to man. Virus Life December:2–5 [Google Scholar]
- 27. Holmes GP, et al. 1990. B virus (Herpesvirus simiae) infection in humans: epidemiologic investigation of a cluster. Ann. Intern. Med. 112:833–839 [DOI] [PubMed] [Google Scholar]
- 28. Howell DM, Feldman M, Siegal FP, Pettera L, Fitzgerald-Bocarsly P. 1993. Peripheral blood of AIDS patients contains cells capable of providing accessory function for the natural killer cell-mediated, lysis of herpes simplex virus-infected targets despite low interferon-alpha production. J. Acquir. Immune Defic. Syndr. 6:15–23 [PubMed] [Google Scholar]
- 29. Huard B, Früh K. 2000. A role for MHC class I down-regulation in NK cell lysis of herpes virus-infected cells. Eur. J. Immunol. 30:509–515 [DOI] [PubMed] [Google Scholar]
- 30. Hurt RD, Meléndez LV. 1969. Herpes virus infection in nonhuman primates: a review. Lab. Anim. Care 19:221–234 [PubMed] [Google Scholar]
- 31. Ishitani A, Geraghty DE. 1992. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc. Natl. Acad. Sci. U. S. A. 89:3947–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jones-Engel L, et al. 2006. Temple monkeys and health implications of commensalism, Kathmandu, Nepal. Emerg. Infect. Dis. 12:900–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keeble SA. 1960. B virus infection in monkeys. Ann. N. Y. Acad. Sci. 85:960–969 [DOI] [PubMed] [Google Scholar]
- 34. Keeble SA, Christofinis GJ, Wood W. 1958. Natural virus-B infection in rhesus monkeys. J. Pathol. Bacteriol. 76:189–199 [DOI] [PubMed] [Google Scholar]
- 35. Kessler MJ, Hilliard JK. 1990. Seroprevalence of B virus (Herpesvirus simiae) antibodies in a naturally formed group of rhesus macaques. J. Med. Primatol. 19:155–160 [PubMed] [Google Scholar]
- 36. Kirszenbaum M, Moreau P, Gluckman E, Dausset J, Carosella E. 1994. An alternatively spliced form of HLA-G mRNA in human trophoblasts and evidence for the presence of HLA-G transcript in adult lymphocytes. Proc. Natl. Acad. Sci. U. S. A. 91:4209–4213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koppers-Lalic D, et al. 2005. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc. Natl. Acad. Sci. U. S. A. 102:5144–5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koppers-Lalic D, et al. 2003. Bovine herpesvirus 1 interferes with TAP-dependent peptide transport and intracellular trafficking of MHC class I molecules in human cells. Arch. Virol. 148:2023–2037 [DOI] [PubMed] [Google Scholar]
- 39. Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. 1998. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J. Immunol. 160:4951–4960 [PubMed] [Google Scholar]
- 40. Lee N, et al. 1998. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. U. S. A. 95:5199–5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le Gal FA, et al. 1999. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 11:1351–1356 [DOI] [PubMed] [Google Scholar]
- 42. LeMaoult J, Zafaranloo K, Le Danff C, Carosella ED. 2005. HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J. 19:662–664 [DOI] [PubMed] [Google Scholar]
- 43. Lo Monaco E, et al. 2008. HLA-E: strong association with beta2-microglobulin and surface expression in the absence of HLA class I signal sequence-derived peptides. J. Immunol. 181:5442–5450 [DOI] [PubMed] [Google Scholar]
- 44. Loomis MR, O'Neill T, Bush M, Montali RJ. 1981. Fatal herpesvirus infection in patas monkeys and a black and white colobus monkey. J. Am. Vet. Med. Assoc. 179:1236–1239 [PubMed] [Google Scholar]
- 45. Lopez C, et al. 1983. Correlation between low natural killing of fibroblasts infected with herpes simplex virus type 1 and susceptibility to herpesvirus infections. J. Infect. Dis. 147:1030–1035 [DOI] [PubMed] [Google Scholar]
- 46. Menier C, Riteau B, Dausset J, Carosella ED, Rouas-Freiss N. 2000. HLA-G truncated isoforms can substitute for HLA-G1 in fetal survival. Hum. Immunol. 61:1118–1125 [DOI] [PubMed] [Google Scholar]
- 47. Morandi F, et al. A novel mechanism of soluble HLA-G mediated immune modulation: downregulation of T cell chemokine receptor expression and impairment of chemotaxis. PLoS One 5:e11763 doi:10.1371/journal.pone.0011763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moreau P, et al. 1995. Soluble HLA-G molecule. An alternatively spliced HLA-G mRNA form candidate to encode it in peripheral blood mononuclear cells and human trophoblasts. Hum. Immunol. 43(3):231–236 [DOI] [PubMed] [Google Scholar]
- 49. Neumann L, Kraas W, Uebel S, Jung G, Tampe R. 1997. The active domain of the herpes simplex virus protein ICP47: a potent inhibitor of the transporter associated with antigen processing. J. Mol. Biol. 272:484–492 [DOI] [PubMed] [Google Scholar]
- 50. Orange JS. 2002. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 4:1545–1558 [DOI] [PubMed] [Google Scholar]
- 51. Orcutt RP, Pucak GJ, Foster HL, Kilcourse JT, Ferrell T. 1976. Multiple testing for the detection of B virus antibody in specially handled rhesus monkeys after capture from virgin trapping grounds. Lab. Anim. Sci. 26:70–74 [PubMed] [Google Scholar]
- 52. Palmer AE. 1987. B virus, Herpesvirus simiae: historical perspective. J. Med. Primatol. 16:99–130 [PubMed] [Google Scholar]
- 53. Park B, et al. 2002. The MHC class I homolog of human cytomegalovirus is resistant to down-regulation mediated by the unique short region protein (US)2, US3, US6, and US11 gene products. J. Immunol. 168:3464–3469 [DOI] [PubMed] [Google Scholar]
- 54. Paul P, et al. 2000. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum. Immunol. 61:1138–1149 [DOI] [PubMed] [Google Scholar]
- 55. Pazmany L, et al. 1996. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science 274:792–795 [DOI] [PubMed] [Google Scholar]
- 56. Perelygina L, et al. 2003. Complete sequence and comparative analysis of the genome of herpes B virus (Cercopithecine herpesvirus 1) from a rhesus monkey. J. Virol. 77:6167–6177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pfänder R, et al. 1999. Structure of the active domain of the herpes simplex virus protein ICP47 in water/sodium dodecyl sulfate solution determined by nuclear magnetic resonance spectroscopy. Biochemistry 38:13692–13698 [DOI] [PubMed] [Google Scholar]
- 58. Pistoia V, Morandi F, Wang X, Ferrone S. 2007. Soluble HLA-G: are they clinically relevant? Semin. Cancer Biol. 17:469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Posavad CM, Rosenthal KL. 1992. Herpes simplex virus-infected human fibroblasts are resistant to and inhibit cytotoxic T-lymphocyte activity. J. Virol. 66:6264–6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rager-Zisman B, Quan PC, Rosner M, Moller JR, Bloom BR. 1987. Role of NK cells in protection of mice against herpes simplex virus-1 infection. J. Immunol. 138:884–888 [PubMed] [Google Scholar]
- 61. Rajagopalan S, Long EO. 1999. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 189:1093–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rappocciolo G, Birch J, Ellis SA. 2003. Down-regulation of MHC class I expression by equine herpesvirus-1. J. Gen. Virol. 84:293–300 [DOI] [PubMed] [Google Scholar]
- 63. Riteau B, et al. 2001. HLA-G1 co-expression boosts the HLA class I-mediated NK lysis inhibition. Int. Immunol. 13:193–201 [DOI] [PubMed] [Google Scholar]
- 64. Riteau B, et al. 2001. Characterization of HLA-G1, -G2, -G3, and -G4 isoforms transfected in a human melanoma cell line. Transplant Proc. 33:2360–2364 [DOI] [PubMed] [Google Scholar]
- 65. Riteau B, et al. 2001. HLA-G2, -G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J. Immunol. 166:5018–5026 [DOI] [PubMed] [Google Scholar]
- 66. Ritz U, et al. 2003. Impaired transporter associated with antigen processing (TAP) function attributable to a single amino acid alteration in the peptide TAP subunit TAP1. J. Immunol. 170:941–946 [DOI] [PubMed] [Google Scholar]
- 67. Shiroishi M, et al. 2003. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc. Natl. Acad. Sci. U. S. A. 100:8856–8861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tomazin R, et al. 1996. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 15:3256–3266 [PMC free article] [PubMed] [Google Scholar]
- 69. Ulbrecht M, et al. 2003. HCMV glycoprotein US6 mediated inhibition of TAP does not affect HLA-E dependent protection of K-562 cells from NK cell lysis. Hum. Immunol. 64:231–237 [DOI] [PubMed] [Google Scholar]
- 70. Urosevic M, Dummer R. 2002. HLA-G—an ace up the sleeve? ASHI Q. 2002:106–109 www.smfn.unisi.it/smfn_lauree/view_matdid.php?id=3098 [Google Scholar]
- 71. Weigler BJ. 1992. Biology of B virus in macaque and human hosts: a review. Clin. Infect. Dis. 14:555–567 [DOI] [PubMed] [Google Scholar]
- 72. Weigler BJ, et al. 1993. Epidemiology of cercopithecine herpesvirus 1 (B virus) infection and shedding in a large breeding cohort of rhesus macaques. J. Infect. Dis. 167:257–263 [DOI] [PubMed] [Google Scholar]
- 73. Wilson RB, Holscher MA, Chang T, Hodges JR. 1990. Fatal Herpesvirus simiae (B virus) infection in a patas monkey (Erythrocebus patas). J. Vet. Diagn. Invest. 2:242–244 [DOI] [PubMed] [Google Scholar]
- 74. Yewdell JW, Bennink JR. 1999. Mechanisms of viral interference with MHC class I antigen processing and presentation. Annu. Rev. Cell Dev. Biol. 15:579–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. York IA, et al. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525–535 [DOI] [PubMed] [Google Scholar]
- 76. Zeitlyonok NA, Chumakova MY, Ralph NM, Lo Siauw G. 1966. Distribution area and natural hosts of latent viruses of monkeys. Occurrence of simian vacuolating virus (SV40) and Herpesvirus simiae in cynomolgus monkeys in Indonesia. Acta Virol. 10:537–541 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.