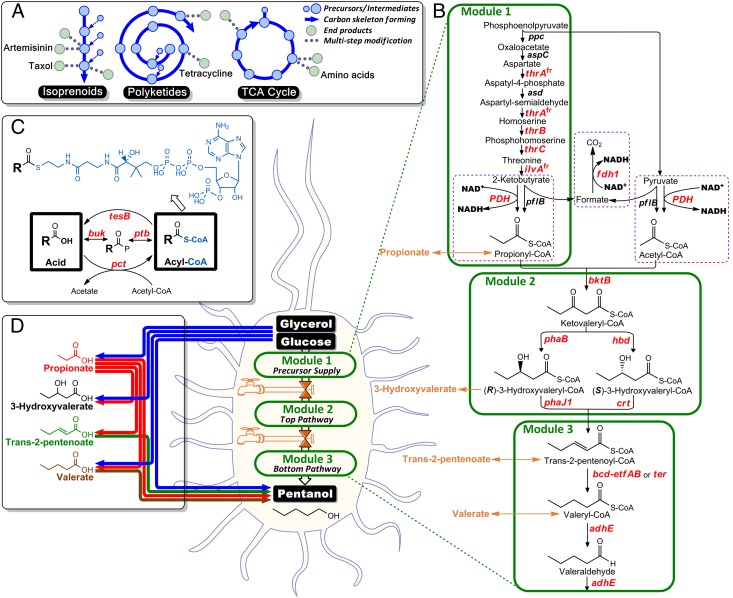
Fig. 1.
Metabolic pathway construction for direct microbial synthesis of pentanol from glucose or glycerol. (A) Nature has evolved an efficient way to supply structurally diverse compounds by initially forming a carbon skeleton from which a handful of bioactive compounds are generated. For example, the highly functionalized antimalarial drug Artemisinin and anticancer drug Taxol can be derived from the isoprenoids pathway; various antibiotics such as Tetracycline can be synthesized from the polyketides pathway; and precursors for synthesis of various amino acids can be supplied from the tricarboxylic acid cycle (TCA). (B) The pentanol biosynthetic pathway consists of three modules, each of which was validated separately and then assembled together. Genes in red and italics were overexpressed from inducible plasmids. (C) An enzyme toolkit was established and used to analyze the pentanol biosynthetic pathway by channeling membrane-impermeable CoA-tagged metabolite intermediates to membrane-permeable free acids, allowing for extracellular detection by HPLC. Similarly, certain free carboxylic acids can be activated with an adduct of a CoA tag using the enzyme toolkit and channeled into the pentanol pathway. (D) Tailor-made synthesis of various odd-chain chemicals can be achieved through particular combinations of the pathway and toolkit enzymes.