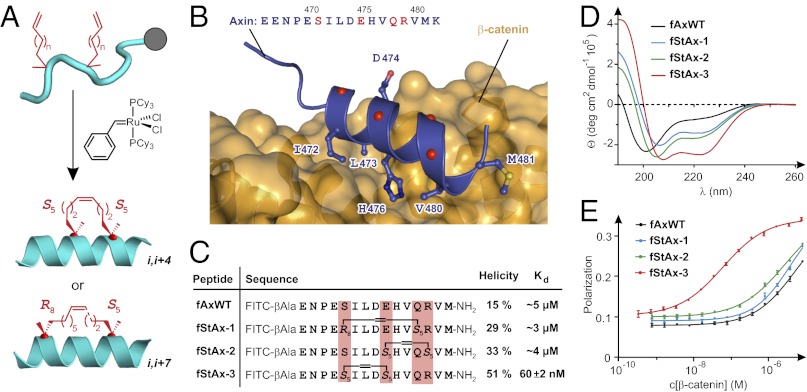
Fig. 2.
Axin-derived stapled peptides. (A) Peptide stapling: Olefin-modified amino acids are incorporated at two positions in the peptide sequence at residues having the relative phasing i,i+4 or i,i+7 and are cross-linked by ruthenium-mediated olefin metathesis. (B) Crystal structure of β-catenin bound to Axin CBD (PDB ID 1QZ7) (18) with the side-chains of interacting residues shown explicitly and the α-carbons of noninteracting residues of the Axin α-helix shown as red spheres. (C) Sequences, helicities, and dissociation constants (Kd, mean ± SE) of Axin-derived FITC-labeled peptides. (D) Circular dichroism spectra of peptides. Details on calculation of α-helicity are provided in SI Methods. (E) FP assay of fluorescein-labeled peptides binding to β-catenin.