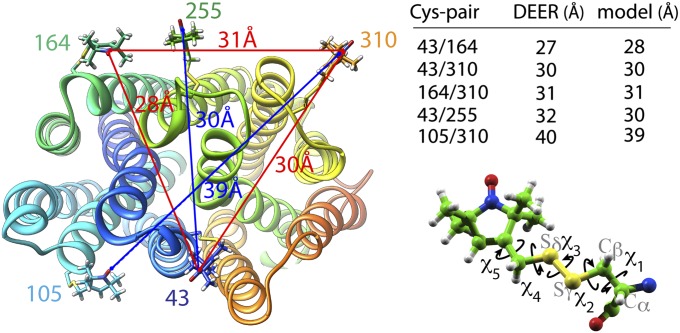
Fig. 4.
Distance trilateration. Nitroxide labels attached to paired Cys replacements are modeled on the proposed apo-intermediate and optimized for clashes with the protein surface (Left). Conformational space was explored by varying the χ-angle (Lower Right) to find conformations of nitroxide side chains that satisfy DEER distances between three residues (Upper Right) [i.e., residues 43–164, 164–310, and 43–310, indicated with red lines (Left)] as described in Experimental Procedures. The next residue (i.e., residue 255) was modeled, and the next trilateration set is optimized in agreement with the DEER data. The distances are taken from a study by Smirnova et al. (18) and SI Appendix, Table S2.