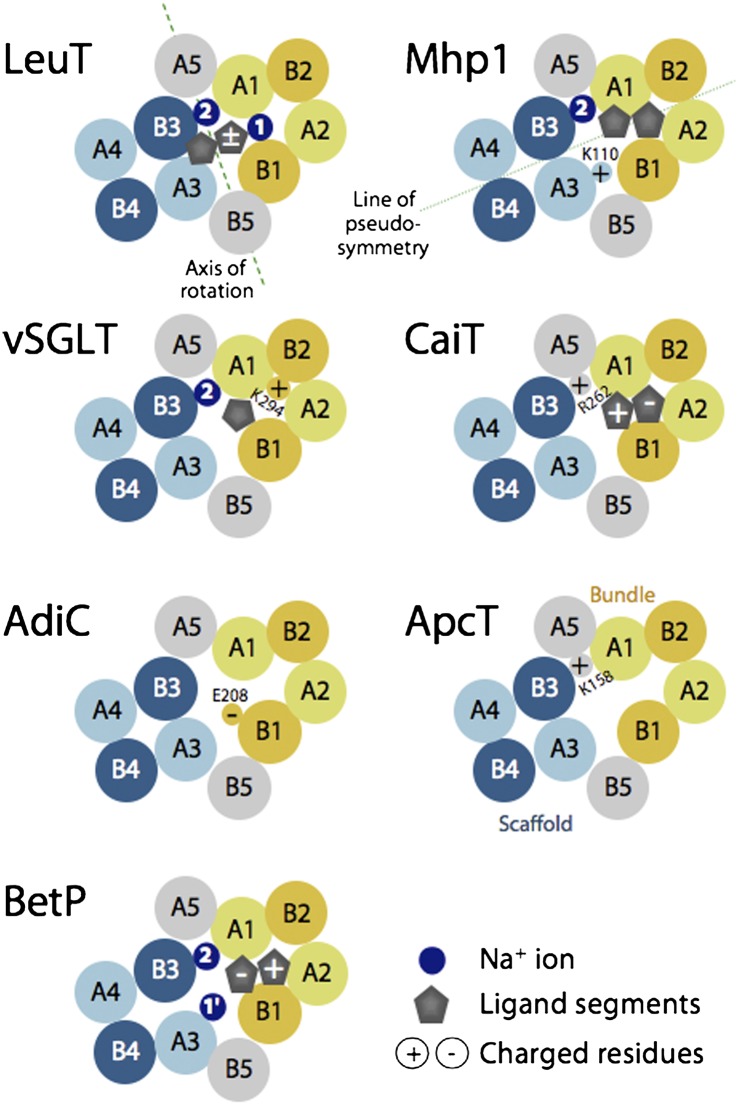
Fig. 1.
Schematics of helix packing in FIRL-fold transporters of known structure, viewed from the periplasm at a slice through the membrane plane roughly midway across the membrane. The approximate locations of ligands (gray symbols), known or proposed sodium-binding sites (dark blue circles), and selected positively or negatively charged amino acids (circles containing a + or − sign, respectively) are shown. A putative axis of rotation (dashed green line) and the axis of pseudosymmetry (dotted green line) are shown. Bundle helices (A1–2 and B1–2) are colored yellow; scaffold helices (A3–5, and B3–5) are colored blue (for the hash domain) or gray (for the arms). Helices in the first repeat are colored lighter shades. See Fig. S1 for the helix numbering in each family.