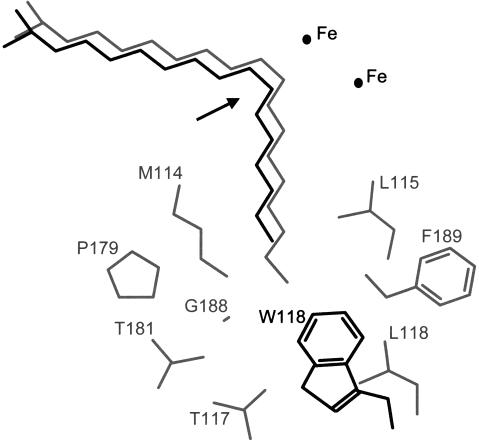
Figure 4.
A model of 18:0 (gray) and 16:0 (black) bound to the active site of acyl-ACP desaturases. The structure of seven common residues lining the substrate pocket of the castor and cat's claw enzymes and L118 of the castor enzyme are shown in gray. The W118 residue of the cat's claw enzyme and the L118W castor mutant is shown in black. The position of the catalytic di-iron center is indicated and the arrow shows the position of double-bond insertion. Amino acid numbering is given with respect to the sequence of the mature castor Δ9–18:0-ACP desaturase.