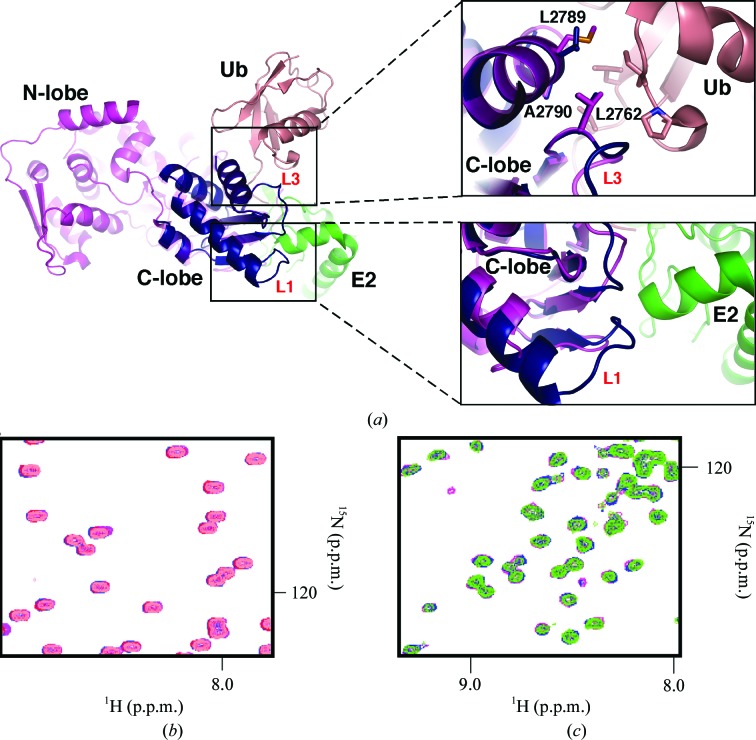
Figure 2.
UBR5 HECT C-lobe does not interact with ubiquitin or UBCH4. (a) The overlay shows how the C-lobe is surrounded by the N-lobe, ubiquitin (Ub) and UbcH5B (E2). The unique conformation of loop L1 in UBR5 (dark blue) relative to Nedd4L (magenta; PDB entry 2oni; Structural Genomics Consortium, unpublished work) suggests that UBR5 makes additional contacts with E2 enzymes. Loop L3 contains a conserved Leu (Leu2762 in UBR5) residue that helps to stabilize the interaction with ubiquitin in the UbcH5B–ubiquitin–HECTNedd4L ternary complex; two further residues in helix α4 (Ala2790 and Leu2789 in UBR5) are also conserved. All of these residues are highly conserved in the family of HECT E3 ligases. (b) Overlay and expansion of 15N–1H correlation spectra of 0.15 mM ubiquitin (salmon) titrated with unlabelled UBR5 HECT C-lobe (maximum concentration of 0.5 mM; purple). No chemical shift perturbations were observed at the maximum concentration of titrant used, which indicates no binding. (c) Overlay and expansion of 15N–1H correlation spectra of 0.15 mM UBCH4 (green) titrated with unlabelled UBR5 HECT C-lobe (maximum concentration of 0.5 mM; purple). No chemical shift perturbations were observed, which indicates an absence of binding.