Conditions such as stroke and spinal cord injury (SCI) reduce the ability to activate muscle and produce movement, typically affecting a patient’s functional capacity and ability to carry out the tasks of daily living. Mobility limitations increase the risk of other avoidable chronic conditions such as cardiovascular disease, diabetes, cancer, osteoporosis, and depression, with dramatic negative effects on the overall quality of life. Appropriately prescribed exercise programs are needed to improve health status and overall quality of life for such individuals.1–3 Most authors now agree that moderate physical activity (PA) is well tolerated by such patients, and it seems to have few adverse side effects or contraindications when compared with common pharmacologic interventions.4 There are approximately 50 000 cerebrovascular accidents in Canada each year,5 and although stroke is only the third leading cause of death in North America, it is the leading cause of disability.6 There are more than 85 000 Canadians with SCI, and this number is projected to increase at a rate of approximately 3000 per year.7 Management of these conditions is thus a priority for today’s family physician.
A large number of stroke and SCI survivors who could benefit greatly from a suitable program of PA are barred from participation because of incorrect perceptions about their functional capacity and undue concern about causing further harm. Commonly expressed concerns are exacerbation of spasticity and contractures, perceived inability to perform basic movements safely, and a substantial risk of falling. The notion that exercise (particularly resistance training) might exacerbate spasticity has now been refuted,8,9 and there is good evidence that individually tailored resistance or aerobic exercise programs can be performed safely after stroke or SCI.10–14 If such programs are not initiated, the reduced ability for movement and perceived barriers to exercise commonly lead to a progressive decrease in the individual’s overall PA, with all the adverse effects of concomitant physical deconditioning.
This article provides an executive summary of findings from a systematic review of the risks of PA in stroke and SCI.15 It was undertaken as one in a comprehensive series of reviews examining the risks of PA in various chronic diseases. The evidence thus obtained provides the foundation for new tools to be used in exercise clearance: the revised Physical Activity Readiness Questionnaire (PAR-Q+) and the electronic Physical Activity Readiness Medical Examination (ePARmed-X+) procedure.16 We briefly discuss available data on the risks of PA in stroke and SCI, and present decision trees that facilitate the family physician’s task of setting appropriate PA prescription and guidelines for the ongoing monitoring of patients.
Discussion
Stroke
In patients with stroke, adverse events are very rare (0.03%) during aerobic training, and they seem nonexistent during resistance training.15 All reported events have been cardiovascular in nature (stroke exacerbation or myocardial infarction); even with such occurrences, it has been difficult to establish a direct cause-and-effect relationship to PA owing to delays in many of the events (eg, 2 days after the trial) or a similar frequency of adverse events in the control group. Nevertheless, the low rate of adverse incidents must be tempered by a recognition that in most studies strict prescreening protocols excluded those at the highest risk of cardiovascular problems. Another limitation in the current evidence base is that most studies used a relatively low exercise intensity (often insufficient to augment physical fitness), owing to preconceived safety concerns or limited physiologic monitoring of participants while they were exercising.
Spinal cord injury
The prevalence of adverse events during PA in patients with SCIs ranged from 0% to 4%.15 The events reported were generally minor in nature (tendon or joint pain), and no cardiovascular incidents were identified. As in the stroke literature, study participants tended to be prescreened for cardiovascular risk factors, including autonomic dysfunction, and the apparent safety of programs must be interpreted with this in mind.
Exercise prescription
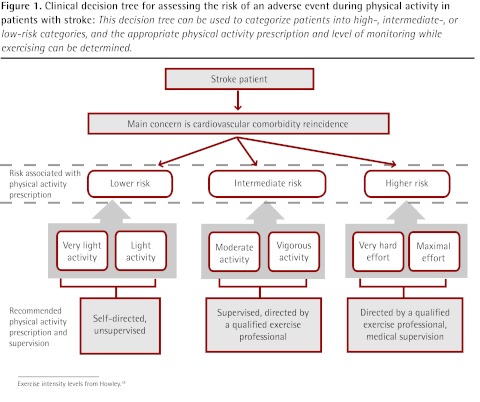
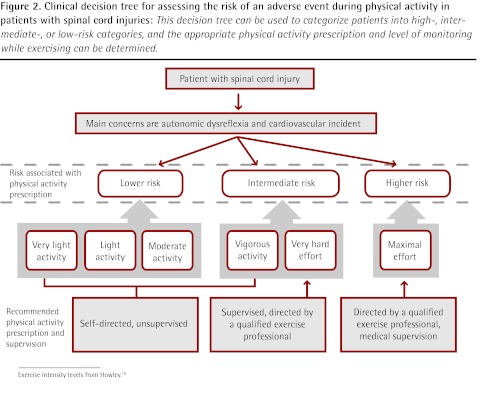
Based on the literature reviewed, the empirical risk-to-benefit ratio clearly favours the recommendation of exercise for both stroke and SCI patients, although screening for potential important risk factors is warranted. In both groups of patients, the greatest risk during PA is the occurrence or reoccurrence of cardiovascular or cerebrovascular events. Because of this attendant risk and probable comorbidity in stroke patients, preliminary exercise testing with electrocardiogram monitoring has been recommended for such patients.17 Given the impracticality of providing electrocardiogram stress testing for all stroke patients, lower volume and reduced intensity of PA in conjunction with thorough prescreening1,17 are suggested when stress testing is not available. The risks of PA for patients with low-level SCI are primarily associated with hypokinetic comorbidities (particularly cardiovascular disease and osteoporosis). In those with high-level lesions, such problems might be compounded by autonomic dysreflexia.12 Our PA recommendations (Table 1) reflect these concerns, providing a schematic for classifying risk and thus determining an appropriate level of supervision for testing and prescribed exercise in both stroke (Figure 1) and SCI (Figure 2).18 In this context, a qualified exercise professional is any member of the allied health care team who has been trained specifically in the monitoring and prescription of PA for those with known chronic disease; in Canada, one example would be a Canadian Society for Exercise Physiology Certified Exercise Physiologist.
Table 1.
Evidence-based recommendations for PA screening in stroke and SCI patients
| Recommendation | Level* | Grade† |
|---|---|---|
| PA performed during the acute stage (< 6 mo from injury onset) of a stroke or SCI must be specifically cleared by the appropriate subspecialist, and patients should only engage in PA under the direct supervision of a QEP | III | A |
| Owing to the risk of cardiovascular comorbidity and underlying pathophysiology, persons with chronic stroke should only perform vigorous PA under the supervision of a QEP | III | A |
| Subspecialist screening is explicitly needed for those with stroke and cardiovascular comorbidities and those with complete SCI at T12 or higher and incomplete SCI at T6 or higher owing to concern about autonomic dysreflexia; exercise in this group should only be performed under the supervision of a QEP | IV | B |
PA—physical activity, QEP—qualified exercise professional, SCI—spinal cord injury.
Level I evidence includes randomized controlled trials; level II evidence includes randomized controlled trials with important limitations or observational trials with overwhelming evidence; level III evidence includes observational trials; and level IV evidence includes anecdotal evidence or expert opinion.
Grade A recommendations are strong; grade B recommendations are intermediate; and grade C recommendations are weak.
Figure 1.
Clinical decision tree for assessing the risk of an adverse event during physical activity in patients with stroke: This decision tree can be used to categorize patients into high-, intermediate-, or low-risk categories, and the appropriate physical activity prescription and level of monitoring while exercising can be determined.
Exercise intensity levels from Howley.18
Figure 2.
Clinical decision tree for assessing the risk of an adverse event during physical activity in patients with spinal cord injuries: This decision tree can be used to categorize patients into high-, intermediate-, or low-risk categories, and the appropriate physical activity prescription and level of monitoring while exercising can be determined.
Exercise intensity levels from Howley.18
Conclusion
Current evidence suggests that PA participation has a favourable risk-to-benefit ratio for most patients with stroke or SCI. However, it is essential that a qualified professional provide appropriate prescreening, exercise prescription, and subsequent PA monitoring. Specific recommendations for PA and its supervision should be derived from individualized risk classification, based on the decision trees that have now been developed.
Footnotes
Competing interests
None declared
References
- 1.Ivey FM, Hafer-Macko CE, Macko RF. Exercise training for cardiometabolic adaptation after stroke. J Cardiopulm Rehabil Prev. 2008;28(1):2–11. doi: 10.1097/01.HCR.0000311501.57022.a8. [DOI] [PubMed] [Google Scholar]
- 2.Pang MY, Eng JJ, Dawson AS, Gylfadottir S. The use of aerobic exercise training in improving aerobic capacity in individuals with stroke: a meta-analysis. Clin Rehabil. 2006;20(2):97–111. doi: 10.1191/0269215506cr926oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noreau L, Shephard RJ. Spinal cord injury, exercise, and quality of life. Sports Med. 1995;20(4):226–50. doi: 10.2165/00007256-199520040-00003. [DOI] [PubMed] [Google Scholar]
- 4.Khadilkar A, Ottawa Panel. Phillips K, Jean N, Lamothe C, Milne S, et al. Ottawa panel evidence-based clinical practice guidelines for post-stroke rehabilitation. Top Stroke Rehabil. 2006;13(2):1–269. doi: 10.1310/3TKX-7XEC-2DTG-XQKH. [DOI] [PubMed] [Google Scholar]
- 5.Heart and Stroke Foundation [website] Statistics. Stroke. Ottawa, ON: Heart and Stroke Foundation; 2011. Available from: www.heartandstroke.com/site/c.ikIQLcMWJtE/b.3483991/k.34A8/Statistics.htm#stroke. Accessed 2012 Sep 18. [Google Scholar]
- 6.Kelly BM, Pangilinan PH, Jr, Rodriguez GM. The stroke rehabilitation paradigm. Phys Med Rehabil Clin N Am. 2007;18(4):631–50. v. doi: 10.1016/j.pmr.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Farry A, Baxter D. The incidence and prevalence of spinal cord injury in Canada: overview and estimates based on current evidence. Vancouver, BC: Rick Hansen Institute, Urban Futures; 2010. Available from: http://fecst.inesss.qc.ca/fileadmin/documents/photos/LincidenceetlaprevalencedestraumamedullaireauCanada.pdf. Access 2012 Sep 24. [Google Scholar]
- 8.Morris SL, Dodd KJ, Morris ME. Outcomes of progressive resistance strength training following stroke: a systematic review. Clin Rehabil. 2004;18(1):27–39. doi: 10.1191/0269215504cr699oa. [DOI] [PubMed] [Google Scholar]
- 9.Patten C, Lexell J, Brown HE. Weakness and strength training in persons with post-stroke hemiplegia: rationale, method, and efficacy. J Rehabil Res Dev. 2004;41(3A):293–312. doi: 10.1682/jrrd.2004.03.0293. [DOI] [PubMed] [Google Scholar]
- 10.Turbanski S, Schmidtbleicher D. Effects of heavy resistance training on strength and power in upper extremities in wheelchair athletes. J Strength Cond Res. 2010;24(1):8–16. doi: 10.1519/JSC.0b013e3181bdddda. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs PL. Effects of resistance and endurance training in persons with paraplegia. Med Sci Sports Exerc. 2009;41(5):992–7. doi: 10.1249/MSS.0b013e318191757f. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004;34(11):727–51. doi: 10.2165/00007256-200434110-00003. [DOI] [PubMed] [Google Scholar]
- 13.Tang A, Sibley KM, Thomas SG, Bayley MT, Richardson D, McIlroy WE, et al. Effects of an aerobic exercise program on aerobic capacity, spatiotemporal gait parameters, and functional capacity in subacute stroke. Neurorehabil Neural Repair. 2009;23(4):398–406. doi: 10.1177/1545968308326426. [DOI] [PubMed] [Google Scholar]
- 14.Brurok B, Helgerud J, Karlsen T, Leivseth G, Hoff J. Effect of aerobic high-intensity hybrid training on stroke volume and peak oxygen consumption in men with spinal cord injury. Am J Phys Med Rehabil. 2011;90(5):407–14. doi: 10.1097/PHM.0b013e31820f960f. [DOI] [PubMed] [Google Scholar]
- 15.Zehr EP. Evidence-based risk assessment and recommendations for physical activity clearance: stroke and spinal cord injury. Appl Physiol Nutr Metab. 2011;36(Suppl 1):S214–31. doi: 10.1139/h11-055. [DOI] [PubMed] [Google Scholar]
- 16.Warburton DER, Jamnik VK, Bredin SSD, Gledhill N. The Physical Activity Readiness Questionnaire (PARQ+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+) Health Fitness J Can. 2011;4(2):3–23. [Google Scholar]
- 17.Gordon NF, Gulanick M, Costa F, Fletcher G, Franklin BA, Roth EJ, et al. Physical activity and exercise recommendations for stroke survivors: an American Heart Association scientific statement from the Council on Clinical Cardiology, Subcommittee on Exercise, Cardiac Rehabilitation, and Prevention; the Council on Cardiovascular Nursing; the Council on Nutrition, Physical Activity, and Metabolism; and the Stroke Council. Circulation. 2004;109(16):2031–41. doi: 10.1161/01.CIR.0000126280.65777.A4. [DOI] [PubMed] [Google Scholar]
- 18.Howley ET. Type of activity: resistance, aerobic and leisure versus occupational physical activity. Med Sci Sports Exerc. 2001;33(6 Suppl):S364–20. doi: 10.1097/00005768-200106001-00005. [DOI] [PubMed] [Google Scholar]