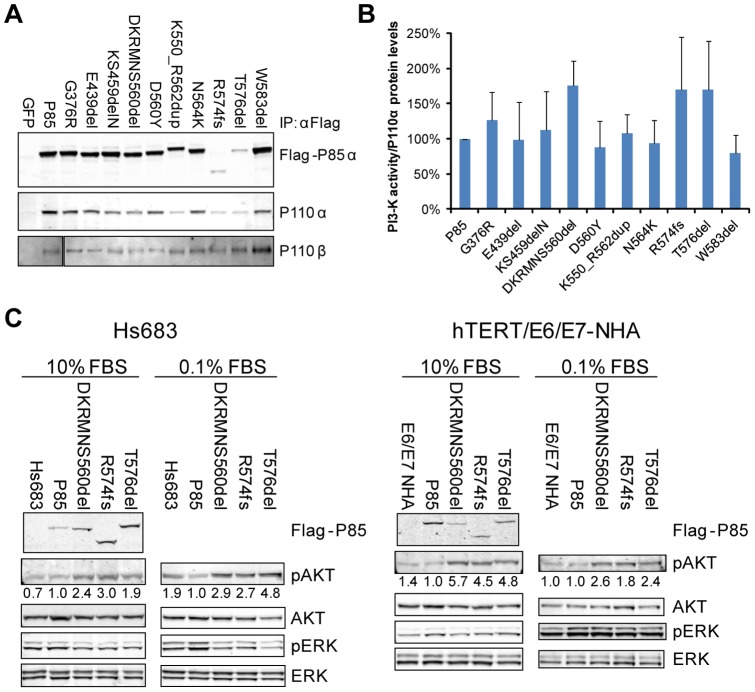
Figure 1. Mutant P85α bound P110α and P110β and increased signaling through the PI3K pathway.
(A) GFP, or Flag-tagged wildtype or mutant P85α was expressed in 293T cells and whole cell lysates immunoprecipitated with anti-Flag antibody. Western blotting demonstrated that mutant P85α constructs retained their interaction with both P110α and P110β. (B) Wildtype or mutant P85α was co-expressed in 293T cells with wildtype P110α, and PI3K heterodimers were immunoprecipitated using anti-Flag antibody. In vitro kinase activity was assessed by measuring phosphorylation of phosphatidylinositol, and total PIP3 signal was quantified and normalized to the total amount of P110α protein loaded in the assay. The average activity from five independent samples is shown (± SD). (C) Expression of mutant P85α constructs increased signaling through the PI3K pathway. Wildtype or mutant P85α was co-expressed with wildtype P110α in Hs683 glioma cells or E6/E7/hTERT-immortalized normal human astrocytes. The resulting cell lines were grown in the indicated concentrations of serum and western blotting was performed to assess activity of the PI3K and MAPK pathways. Representative western blots from at least three experiments are shown. Numerical values below each pAKT panel of the immunoblots represent quantification of the relative protein level by densitometry (normalized to AKT).