Abstract
In monolayer culture, primary articular chondrocytes have an intrinsic tendency to lose their phenotype during expansion. The molecular events underlying this chondrocyte dedifferentiation are still largely unknown. Several transcription factors are important for chondrocyte differentiation. The Ets transcription factor family may be involved in skeletal development. One family member, the Erg gene, is mainly expressed during cartilage formation. To further investigate the potential role of Erg in the maintenance of the chondrocyte phenotype, we isolated and cultured chondrocytes from the rib cartilage of embryos of transgenic mice that express a dominant negative form of Erg (DN-Erg) during cartilage formation. DN-Erg expression in chondrocytes cultured for up to 20 days did not affect the early dedifferentiation usually observed in cultured chondrocytes. However, lipid droplets accumulated in DN-Erg chondrocytes, suggesting adipocyte emergence. Transcriptomic analysis using a DNA microarray, validated by quantitative RT-PCR, revealed strong differential gene expression, with a decrease in chondrogenesis-related markers and an increase in adipogenesis-related gene expression in cultured DN-Erg chondrocytes. These results indicate that Erg is involved in either maintaining the chondrogenic phenotype in vitro or in cell fate orientation. Along with the in vitro studies, we compared adipocyte presence in wild-type and transgenic mice skeletons. Histological investigations revealed an increase in the number of adipocytes in the bone marrow of adult DN-Erg mice even though no adipocytes were detected in embryonic cartilage or bone. These findings suggest that the Ets transcription factor family may contribute to the homeostatic balance in skeleton cell plasticity.
Introduction
Chondrocytes, osteoblasts, fibroblasts, adipocytes and skeletal myoblasts are highly specific cell types derived from multipotent mesenchymal stem cells (MSC) through a specific differentiation pathway [1], [2], [3], [4], [5]. Although MSCs are fully committed to a developmental lineage, several studies have shown that MSC-derived cells can switch to another cell lineage or return to an uncommitted developmental stage [6], [7], [8], [9], [10]. Likewise, the signalling molecules and pathways leading to transdifferentiation (i.e. lineage reprogramming) remain poorly defined.
Chondrogenesis is a tightly regulated process that is initiated by the condensation of committed MSCs, followed by differentiation into chondrocytes and the expression of cartilage-specific markers [11], [12], [13]. Each specific differentiation program of MSC-derived cell types is harmoniously and dynamically controlled by several specific signal transduction (cytokines, growth factors and extracellular matrix molecules) and transcription factors [14], [15]. The osteochondrogenic state is regulated by two master transcription factors: Sox9 (Sex determining region Y-box9) and Runx2 (Runt-related transcription factor 2) essential for the determination and maturation of chondrocytes and osteoblasts, respectively [16], [17]. Several molecular players have been identified, including the transcription factors NF-κB, C/EBPβ, ETS, Runx2, and hypoxia-inducible factor-2α, all of which are involved in cartilage formation [18], [19], [20]. Among them, we and others have shown that the Ets-related gene (Erg) is expressed during the earliest events of skeletal formation and is associated with precartilaginous condensation and chondrogenic differentiation [21], [22], [23], [24]. The Erg transcription factor belongs to the ETS family of DNA-binding proteins [25]. Several members of the ETS family are involved in a variety of cellular and developmental processes. In skeletal formation, the Erg gene is the earliest ETS member family expressed in cartilage during embryonic development followed by Fli1, Ets-2 and Pea3 in a lesser extend [24], [26]. This family of transcriptional regulators shares a highly conserved 85 amino-acid DNA-binding domain (ETS domain) that specifically recognises DNA over an 11 bp sequence centred around a consensus core sequence, 5′-GGAA/T-3′ [27].
To explore the roles Erg may play in the chondrogenesis process or in chondrogenic maturation, we established a transgenic mouse model that overexpresses a dominant negative fragment of the Erg protein (DN-Erg), specifically restricted to the ETS domain. The transgene construct is specifically expressed in chondrocytes during cartilage formation in embryos because it is under the control of the collagen II (Col2a1) promoter and competes with endogenous wild-type Erg protein functions. However, since other ETS family genes, such as Fli1, Ets2 and Pea3 are also expressed, to a lesser extent, in cartilage, we cannot rule out that the binding of these transcription factors is also involved. Transgenic mouse embryos and newborns have no obvious malformations, but clinical early-ageing processes, including hyperlordosis/hyperkyphosis and reduced mobility, are observed during the first 6 months post natum (unpublished data). This manifestation of early ageing indicates that the Erg transcription factor is involved in the regulation of various genes affecting cartilage formation and skeletogenesis.
In this study, to explore the physiological roles of Erg proteins in the maintenance of the chondrocyte phenotype, we isolated chondrocytes from the rib cage of embryos of wild-type (wt) and DN-Erg transgenic mice and cultured them in monolayers. We observed that transgene expression was correlated with the accumulation of lipid droplets in cultured chondrocytes compared to wt. To determine the differentially expressed gene profile during the dedifferentiation process from monolayer-cultured wt and DN-Erg chondrocytes, we used DNA microarray analysis to study the transcriptome modifications in chondrocytes of wt and DN-Erg transgenic mice during culture. Among functional categories accounting for most genes with altered expression in cultured DN-Erg chondrocytes, the adipocyte pathway genes were upregulated.
In addition, because the phenotype of transgenic mice overexpressing DN-Erg was associated with early-ageing skeleton phenotypes and ageing is associated with decreased bone marrow cellularity and increased bone marrow fat, we performed a histological comparison of morphological features of bone marrow from adult mice femur and showed a dramatic increase in adipocytes in DN-Erg transgenic mice.
The data presented here demonstrate that monolayer-cultured DN-Erg chondrocytes spontaneously underwent adipocyte differentiation, suggesting that Erg is involved in the differentiation plasticity of chondrocytes.
Materials and Methods
Cell cultures
Chondrocytes of murine embryos were isolated from the ribs of 18.5 days post-coitum (E18.5) mice according to the protocol described in [28], [29]. Mouse care and treatment were conducted in accordance with institutional guidelines in compliance with national law and policies. This study was specifically approves by our local ethics committee (Authorisation no. CEEA 13/2009 issued by the Comite d'Ethique en Experimentation Animale, Nord-Pas-de-Calais). Chondrocytes were seeded in 6-well culture plates at 106 cells/well and were grown for 20 days. Cells were cultured in DMEM Nutrient Mixture F-12 Ham (Sigma) supplemented with 10% foetal bovine serum, 1% gentamicin, and 1% glutamine. The culture medium was replaced every two days.
Cytochemical analyses
Alcian blue staining
Cultures were washed with PBS before fixing with methanol for 10 min. After rinsing with PBS, cultures were stained overnight with Alcian blue solution (pH 2.5, 3% glacial acetic acid). Cells were washed three times with glacial acetic acid (4%) and twice with sterile distilled water.
Oil red O staining
Cells were washed twice with PBS and fixed with 10% formalin (pH 7.4) for at least 1 h at room temperature. After washing with 60% isopropanol, the cells were stained for 10 min at room temperature with filtered Oil red O/60% isopropanol solution. The cells were washed twice with distilled water. Red-stained adipocytes were observed under a light microscope.
Histological analysis
Whole skeletons of E18.5 embryos were stained with Alizarin red S and Alcian blue [30]. Ribs and legs of 18.5 day embryos and of 40 week-old mice were dissected, fixed and embedded in paraffin. Paraffin blocks were prepared using standard histological procedures. The resulting serial sections (5–6 µm thickness) were stained with hematoxylin and eosin or Sudan black B, as indicated.
Adipocyte number was quantified by three different observers looking at five different fields per section at least three different mice. The result was expressed as the mean of total adipocyte number per square millimeter of marrow tissue area in the analysed fields.
Immunohistochemistry
According to the provided protocol for immunohistochemistry, sections were demasked by treatment with xylene twice for 5 min at 37°C, rehydrated through graded ethanols and were incubated with rabbit anti-mouse adiponectin Acrp30 (N-20, sc-17044-R from Santa Cruz Biotechnology, dilution 1/100). Antigen retrieval was conducted in sodium citrate buffer pH 6 for 30 minutes at 95°C. A biotinylated goat anti-rabbit IgG antibody (Vector), diluted 1∶1000, was used as the secondary antibody with PBS for 1 hour at room temperature. Antigen immunolocalization was analysed using DAB peroxidise substrate (vector labs) according to manufacturer's instructions. Negative controls were realized by omitting primary antibodies. Counterstaining was done with hematoxylin.
RNA preparation
Total RNA was isolated using the Nucleospin RNA II System (Macherey-Nagel GmbH &Co., Düren, Germany) according to the manufacturer's protocol. The RNA was eluted with 50 µl of RNase-free water. RNA integrity and purity were verified using the Agilent Bioanalyzer system (Agilent Technology).
PCR and quantitative real-time PCR (RT-qPCR)
For PCR, 1 µg of total RNA was first reverse-transcribed using Superscript II reverse transcriptase (Invitrogen), random hexamers (Roche), and dNTPs at 42°C for 1 h. For non-quantitative PCR, the High Fidelity PCR Master kit (Roche) was used according to the manufacturer's protocol. Amplification conditions were adjusted to be within the linear range. Quantitative real-time PCR (RT-qPCR) was performed on a LightCycler (Roche Diagnostics) using the LightCycler FastStart DNA Master SYBR Green kit (Roche Diagnostics) according to the manufacturer's instructions. The PCR primers were designed to amplify cDNA fragments ranging in size from 150 to 400 bp and are listed in Table 1. Gene expression levels in each sample were determined using the comparative Ct method (after validation assays for each gene primer set), using the hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene as an endogenous control. The wt control was set to 1 and expression data are presented as bar graphs of the mean values with their SD.
Table 1. Primer sequences of genes investigated in this study.
| Symbol | Full name | Accession # | Forward (F) and Reverse (R) primer sequence |
| Adamts5 | Disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 5 | NM_011782 | F: 5′-ATGCAGCCATCCTGTTCAC-3′ |
| R: 5′-CATTCCCAGGGTGTCACAT-3′ | |||
| Adipoq | Adiponectin, C1Q and collagen domain containing | NM_009605 | F: 5′-CAGGCATCCCAGGACATCC-3′ |
| R: 5′-CCAAGAAGACCTGCATCTCCTTT-3′ | |||
| Col2a1 | Collagen, type II, alpha 1 | NM_001113515 | F: 5′-GGTGGCTTCCACTTCAGCTAT-3′ |
| R: 5′-TCATTGGAGCCCTGGATGAG-3′ | |||
| Col10a1 | Collagen, type X, alpha 1 | NM_009925 | F: 5′-TTCTCCTACCACGTGCATGTG-3′ |
| R: 5′-AGGCCGTTTGATTCTGCATT-3′ | |||
| CtsC | Cathepsin C | NM_009982 | F: 5′-CCAACTGCACCTACCCTG-3′ |
| R: 5′-CTGAACGGTATTGATGGCCT-3′ | |||
| CtsS | Cathepsin S | NM_021281 | F: 5′-TGGTGACGAAGATGCCCTGAAAGA-3′ |
| R: 5′-TGCCATCAAGAGTCCCATAGCCAA-3′ | |||
| Erg | Avian erythroblastosis virus E-26 (v-ets) oncogene related | NM_133659 | F: 5′-GTGGGCGGTGAAAGAATATGG-3′ |
| R: 5′-CTTTGGACTGAGGGGTGAGG-3′ | |||
| DN-Erg | Transgen Dominant-Negatif Erg | - | F: 5′-ACCCACAGAAGATGAACTTTG-3′ |
| R: 5′-GGATCCACTAGTTCTAGAGG-3′ | |||
| Fabp4 | Fatty acid binding protein 4, adipocyte | NM_024406 | F: 5′-CAAAATGTGTGATGCCTTTGTG-3′ |
| R: 5′-GGCTCATGCCCTTTCATAAAC-3′ | |||
| Hprt | Hypoxanthine guanine phosphoribosyl transferase | NM_013556 | F: 5′-GCTGGTGAAAAGGACCTCT-3′ |
| R: 5′-AAGTAGATGGCCACAGGACT-3′ | |||
| Matr3 | Matrin3 | NM_010770 | F: 5′-TTACCAGCACCCAGATTTCC-3′ |
| R: 5′-TGGAGCAAGTCACAGTCGTC-3′ | |||
| Mmp3 | Matrix metallopeptidase 3 | NM_010809 | F: 5′-TGACCCACATATTGAAGAGC-3′ |
| R: 5′-ACTTGACGTTGACTGGTGTC-3′ | |||
| Mmp9 | Matrix metallopeptidase 9 | NM_013599 | F: 5′-ACTCACACGACATCTTCCAG-3′ |
| R: 5′-AGAAGGAGCCCTAGTTCAAG-3′ | |||
| Mmp13 | Matrix metallopeptidase 13 | NM_008607 | F: 5′-TTTATTGTTGCTGCCCATGA-3′ |
| R: 5′-TTTTGGGATGCTTAGGGTTG-3′ | |||
| Plin | Perilipin 1 | NM_001113471 | F: 5′-TGCTGGATGGAGACCTC-3′ |
| R: 5′-ACCGGCTCCATGCTCCA-3′ | |||
| Pparg | Peroxisome proliferator activated receptor gamma | NM_011146 | F: 5′-GCATCAGGCTTCCACTATGGA-3′ |
| R: 5′-AAGGCACTTCTGAAACCGACA-3′ | |||
| Runx2 | Runt related transcription factor 2 | NM_001145920 | F: 5′-GAGGCCGCCGCACGACAACCG-3′ |
| R: 5′-CTCCGGCCCACAAATCTCAGA-3′ | |||
| Scin | Scinderin | NM_009132 | F: 5′-AACAGTGGTAGAGTCCAGATT-3′ |
| R: 5′-GTGATAGATGCCAGGTTCCTC-3′ | |||
| Sox9 | SRY-box containing gene 9 | NM_011448 | F: 5′-TGGCAGACCAGTACCCGCATCT-3′ |
| R: 5′-TCTTTCTTGTGCTGCACGCGC-3′ |
Microarray Analysis
Hybridisation was carried out following the Two-Colour Microarray-Based Expression Analysis protocol (Agilent Technologies). For each sample, 1 µg of total RNA per sample was divided into two equal aliquots to enable technical replication known, as a dye-swap hybridisation, and amplified. Dye-swap hybridisations were performed by reversing the dyes for each of the RNA samples. The reverse transcription and the labelling were conducted using the protocol recommended by Agilent (Agilent Low RNA Input Fluorescent Linear Amplification kit). Whole Mouse Genome Oligo Microarrays from Agilent Technologies were used and hybridisations were performed for 17 h at 65°C (Gene Expression Hybridization kit, Agilent Technologies). Slides were washed and scanned using Innoscan 700 (Innopsys) and raw data were processed using the Limma package (Linear Models for Microarray Data) running under the statistical language R. A normalisation protocol consisting of a within-array loess normalisation to correct for dye and spatial effects was applied on the median or mean intensities of the spots. After normalisation, identification of statistically significant deregulation was performed using a moderated Student's t-test with empirical Bayes shrinkage of standard errors. We selected genes whose expression was significantly upregulated (mean log-ratio >3.3x; adjusted p-value <0.005) or downregulated (mean log-ratio <−3.3x; significant at the adjusted p-value <0.005 level). The data were further analysed using MultiExperiment Viewer (version 4.5), Fatigo and Pathway-Express. The latter was used to predict molecular signalling pathways by assessing an impact factor that accounts for contribution to the proportion of differentially regulated genes in a given pathway.
Results
Morphological phenotype of adipocytes in monolayer-cultured DN-Erg chondrocytes
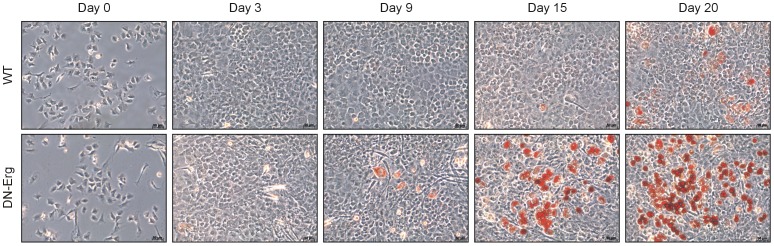
To investigate how modified Erg function (via dominant negative transgene expression) affects chondrocyte phenotype, we isolated chondrocytes from the ribs of wt or DN-Erg E18.5 embryos and cultured them in a conventional monolayer culture. After plating chondrocytes in culture dishes (designated as day 0), cells showed a polygonal shape until confluence (Fig. 1, day 0 to day 3). Thereafter, the morphology of DN-Erg chondrocytes changed rapidly and dramatically. Some DN-Erg cells became enlarged fibroblast-like cells and accumulated vesicles. These vesicles were strongly stained with Oil red O, suggesting they were lipid droplets. Compared with DN-Erg cells, which accumulated lipid droplets from day 9 to day 20 (Fig. 1 and Fig. S1), wt chondrocyte cultures only just begun to show weak Oil red O staining as of day 20. Using an Alcian blue stain as a chondrocyte-specific matrix stain, DN-Erg cultures showed a decrease in cartilage matrix deposition in comparison to wt cultures (Fig. S2). These morphological and cytochemical observations showed that monolayer-cultured DN-Erg chondrocytes display an adipocyte-like phenotype, suggesting that DN-Erg transgene expression favours an adipocyte-like phenotype in monolayer culture over time.
Figure 1. Morphological changes in wt and DN-Erg E18.5 chondrocytes in culture.
Chondrocytes from freshly isolated from ribs of wt and DN-Erg transgenic mouse embryos (at E18.5) were cultured for up 20 days and stained with Oil red O. Phase-contrast images at days 0, 3, 9, 15 and 20 (with day 0, the day of plating) are shown (Scale bar, 50 µm).
Decrease in chondrocyte marker expression and detection of adipocyte-related genes in cultured transgenic DN-Erg chondrocytes
We observed great morphological differences between DN-Erg and wt chondrocytes over the 20 day culture period. To characterize the adipocyte-like phenotype of DN-Erg chondrocytes, we paid special attention to the expression of specific chondrogenic and adipogenic markers using quantitative real-time PCR (Fig. 2). Total RNA was collected from wt and DN-Erg chondrocytes after 0, 6, 10, 15 and 20 days of culture.
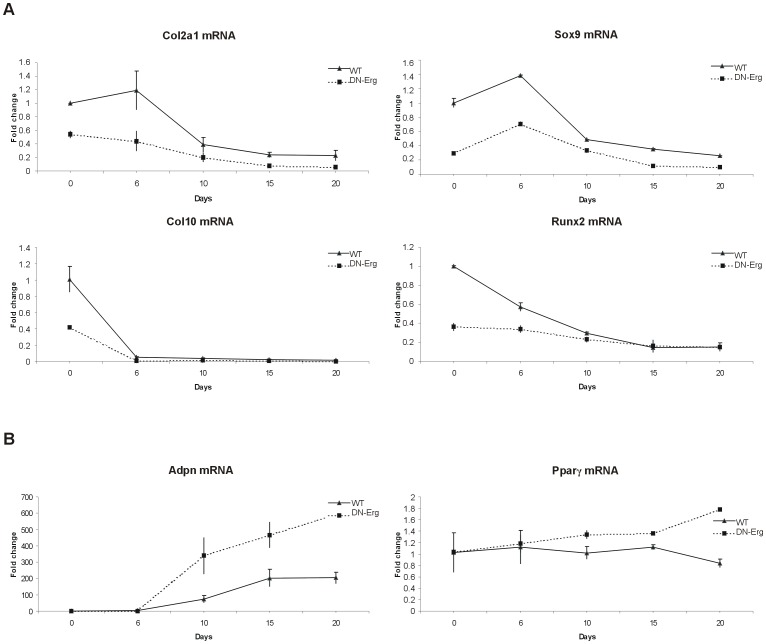
Figure 2. Expression of chondrogenic and adipogenic markers during monolayer culture of wt and DN-Erg chondrocytes.
A. Expression of chondrogenic genes Col2a1, Sox9, Col10, and Runx2. Gene expression was evaluated by RT-qPCR. B. Expression of adipogenic genes Adpn, and Pparγ. Gene expression was evaluated by RT-qPCR. The reported target gene: Hprt transcript ratio in chondrocytes was normalised to the target gene:Hprt transcript ratio (set to 1) of freshly plated wt chondrocytes (day 0). Data represent the mean of at least 2 independent chondrocyte cultures from 2 distinct mice for each genotype.
We studied the expression of four major chondrocyte markers, namely Col2a1, Sox9, Col10 and Runx2 (Fig. 2A). As expected, in wt chondrocytes, Col2a1 and Sox9 showed a peak in expression during the first 6 days of culture, followed by a dramatic decrease. The expression level of these two markers was 2-fold and 4-fold lower, respectively, in DN-Erg chondrocytes compared with wt chondrocytes. Likewise, Col10 and Runx2 expression, which were 2- to 3-fold downregulated in DN-Erg, decreased rapidly in both chondrocyte cultures within 6 and 10 days of culture, respectively, and remained at low levels throughout the rest of the culture period. We also verified DN-Erg expression. As expected, since the transgene is under Col2a1 promoter control, the transgene expression was expressed during the culture, and its pattern was similar to the Col2a1 pattern (Fig. S3).
Because Oil red O staining revealed lipid vesicles in cultured DN-Erg cells, we assayed the expression of adipocyte markers, such as the adiponectin (Adpn) and peroxisome proliferator-activated receptor gamma (Pparγ) genes (Fig. 2B). In both chondrocyte cultures, we detected Adpn transcripts at very low levels until day 6 of culture. Adpn expression was upregulated at day 20 compared to day 0, when mRNA levels were 200- and 600-fold greater in wt and DN-Erg chondrocytes, respectively. Although Pparγ mRNA level remained constant throughout wt chondrocyte culture, it increased in DN-Erg chondrocytes after 6 days of culture.
Taken together, these results validated the observed phenotypic changes. DN-Erg chondrocytes cultured as monolayers experienced a dramatic decrease in the expression of chondrocyte-specific markers and a strong emergence of adipocyte-specific markers.
Comparative differential transcriptomic analysis of monolayer-cultured wt and DN-Erg chondrocytes
To investigate and compare all the modulated genes in monolayer-cultured DN-Erg and wt chondrocytes over time, we monitored the change in genome-wide expression patterns with a global differential approach using whole Mouse Genome Oligo Microarrays (Agilent). Using the monolayer cultures, total RNA isolated from day 0 to day 20 were hybridised to a microarray containing 44,000 probe sets representing nearly 41,000 mouse genes. Comparative microarray analyses were done using Mapix 3.2 (Innopsys). Because the number of differentially expressed genes was high, we chose a cut-off value of a 10-fold change in expression and an adjusted p-value of <0.005.
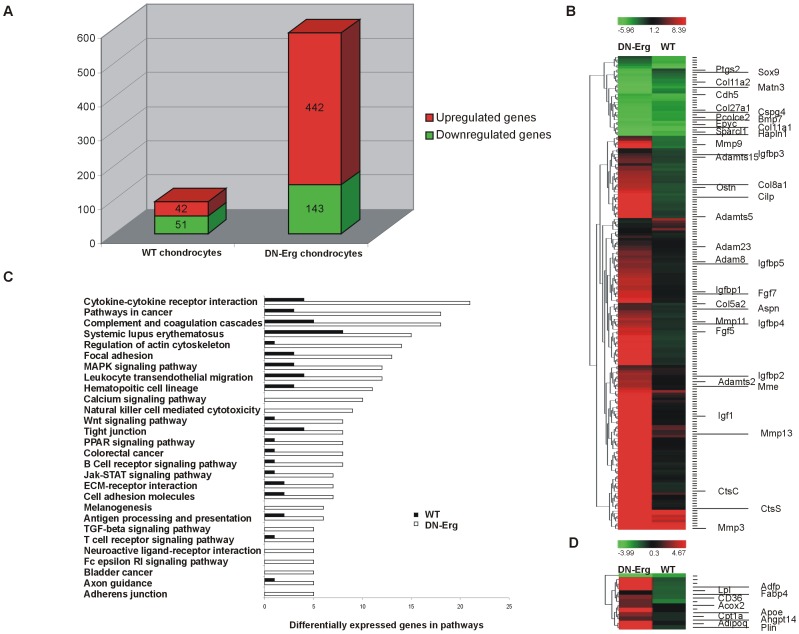
The results show that 93 transcribed genes (42 upregulated and 51 downregulated) were significantly modified 10-fold or more between day 0 and day 20 in cultured wt chondrocytes (Fig. 3A and Table S1), 585 genes (442 upregulated and 143 downregulated) were modified between day 0 and day 20 in cultured DN-Erg chondrocytes (Fig. 3A and Table S2). Finally, even with the stringent cut-off criterion (i.e. value of 10-fold or more), the number of modified genes was six times greater in DN-Erg chondrocytes than in wt chondrocytes between day 0 and day 20 of culture.
Figure 3. Microarray analysis and gene ontology analysis of signalling pathways.
A. Numbers of genes with differential expression between monolayer culture day 0 and day 20 in wt and DN-Erg embryo (E18.5) chondrocytes. Probe sets were filtered according to a 10-fold change cut-off. B. Hierarchical Clustering (HCl) diagram with clusters genes corresponding to the “extracellular matrix”, “metallopeptidase activity”, “Cartilage condensation and development”, “Ossification” annotations. C. Major signalling pathways predicted using Pathway-Express. Pathways listed are pathways with at least 5 or more genes which expression was modified during culture, as determined by Pathway Express. D. Hierarchical Clustering (HCl) tree with clusters of “Lipid metabolism process” and “Lipid transport” genes.
To classify the genes whose expression had been modified, further examination was performed on the basis of Gene Ontology (GO) annotations (Fig. 3B). We first focused on genes classified by the following functional annotations from the Gene Ontology Database: “extracellular matrix region”, “metallopeptidase activity”, “cartilage condensation and development”, and “ossification” annotations. We then used hierarchical clustering to group together genes with similar expression patterns (Table S3). This process resulted in a selection of 190 upregulated or downregulated genes coding for collagens, proteoglycans, matrix-modifying enzymes, growth factors and transcription factors (Fig. 3B). Among these 190 genes, 9 genes whose expression is known to decrease during chondrocyte dedifferentiation were assayed and found to be dramatically downregulated in cultured DN-Erg chondrocytes: scinderin (Scin), hyaluronan and proteoglycan link protein1 (Hapln1), matrilin 3 (Matn3), SPAR like 1, Col11a2, epiphycan (Epyc), col27a1, fibroblast growth factor receptor (Fgfr3) and the transcription factor Sox9, which showed decreased expression in the previous experiment (Fig. 2B). Likewise, the following genes were substantially upregulated: matrix metallopeptidase and aggrecanase (Adamts5, Adamts2, Adamts15, Mmp 13, Mmp3, Mmp9, and Mmp11).
To identify functionally related patterns from the list of genes differentially expressed between day 0 and day 20, we carried out a pathway analysis and compared the relative distribution of functions (Fig. 3C). Genes modified between day 0 and day 20 in DN-Erg chondrocytes were involved in several functional pathways. In accordance with what we observed in the cultured DN-Erg cell plate, we noticed clusters of genes involved in the ‘PPAR signalling pathway’ and in ‘adipocyte differentiation’ (from the Gene Ontology Database) (Table S4). Nine transcripts (Adpn, periplin (Plin), fatty acid binding protein 4 (Fabp4), lipoprotein lipase (Lpl), carnitine palmitoyltransferase 1a liver (Cpt-1a), acyl-Coenzyme A oxidase 2 branched chain (Acox2), angiopoietin-like 4 (Angptl4), CD36 antigen (CD36), adipose differentiation related protein (Adfp)) were associated with the ‘PPAR signalling pathway’ (Fig. S4) and were significantly upregulated in DN-Erg chondrocytes cultured for 20 days (Fig. 3D and Table S4).
Validation of changes in gene expression by real-time PCR
To confirm the observed differences in gene expression between day 0 and day 20 of cultured DN-Erg and wt cells by microarray assay, we performed a quantitative RT-PCR (RT-qPCR) analysis on 11 additional selected genes products (Table 2) in addition to Sox9 and Adpn genes (Fig. 2). In all cases, the direction of change in expression was concordant between the microarray and RT-qPCR results, although absolute values of the microarray-estimated fold change and RT-qPCR-calculated fold change were different (e.g. Matn3). Nevertheless, RT-qPCR results were in line with microarray data and confirmed the loss of the chondrocyte phenotype and the dramatic upregulation of adipocyte markers in cultured DN-Erg chondrocytes.
Table 2. Correlation of microarray data and RT-qPCR analysis.
| Microarray | Real-Time qPCR | ||||
| (Fold-change between day 20 vs day 0 of culture) | (Fold-change between day 20 vs day 0 of culture) | ||||
| Sequence Name | Accession | WT | DN-ERG | WT | DN-ERG |
| Cartilage Development | |||||
| Scinderin | NM_009132 | −2 | −71 | −14 | −795 |
| Matn3 | NM_010770 | −7 | −39 | 1 | −2 |
| Metalloproteinases | |||||
| ADAMTS5 | NM_011782 | 2 | 49 | 4 | 56 |
| MMP3 | NM_010809 | 40 | 284 | 13 | 87 |
| MMP9 | NM_013599 | −1 | 26 | 1 | 137 |
| MMP13 | NM_008607 | 11 | 49 | 10 | 71 |
| Cathepsine | |||||
| CTS C | NM_009982 | 4 | 131 | 39 | 317 |
| CTS S | NM_021281 | 6 | 288 | 52 | 1116 |
| Adipocyte Differentiation | |||||
| Plin | NM_175640 | 4 | 64 | 3 | 16 |
| FABP4 | NM_024406 | 3 | 16 | 146 | 284 |
| Adipoq | NM_009605 | 9 | 84 | 200 | 467 |
Comparison of fold change in the expression of 11 genes implicated in chondrogenesis or adipogenesis as determined by microarray analysis and RT-qPCR.
Fold change between day 0 and day 20 observed by microarray-analysis and RT-qPCR for wt and DN-Erg chondrocytes are shown. Expression levels of target genes obtained by RT-qPCR were normalised to Hprt. Upregulation is indicated by positive values and downregulation is indicated by negative values.
Predominant adipogenesis in bone marrow of adult DN-Erg mice
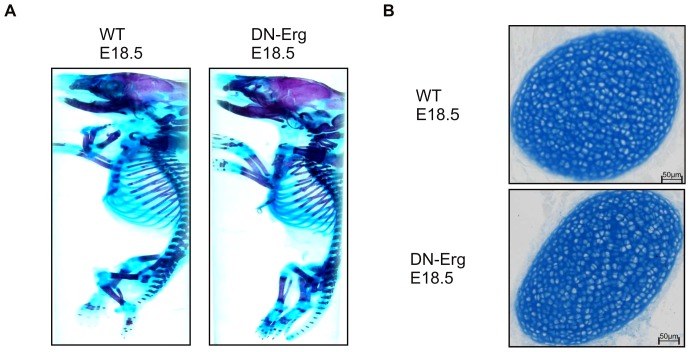
Since the cultured primary chondrocytes were isolated from the ribs of 18.5 day-old wt and DN-Erg embryos, we compared the cartilaginous framework phenotype of wt and DN-Erg embryos (Fig. 4A and unpublished data). The ribs were morphologically homogenous in appearance and consisted only of chondrocytes evenly distributed in extracellular matrix (Fig. 4B).
Figure 4. Histological examination of 18.5 day-old embryos.
A. Skeletal and cartilage preparations of wt (left) and DN-Erg (right) mice at E18.5. Cartilage stained with Alcian blue, bone with Alizarin red. DN-Erg E18.5 embryos did not show any overt abnormalities in cartilaginous or skeletal development. B. Distribution of chondrocytes on sections of rib. Paraffin-embedded sagittal sections of wt (left) and DN-Erg (right) newborn mice were stained with Alcian blue. Bars = 50 µm.
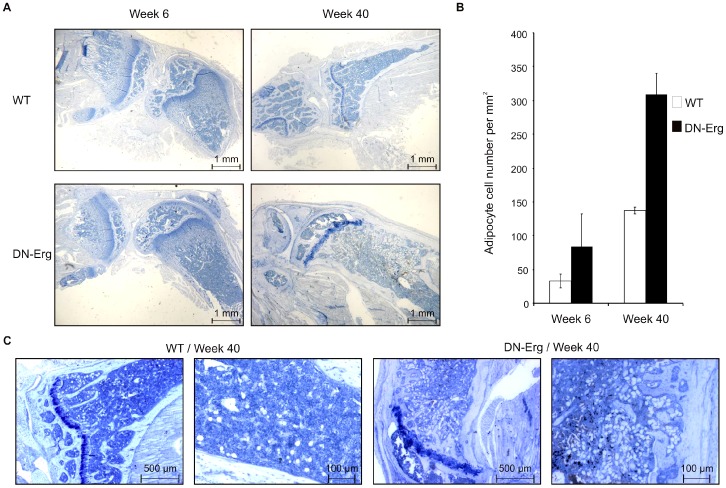
The particular phenotype of DN-Erg transgenic mice, such as early-ageing processes, including hyperlordosis/hyperkyphosis and reduced mobility, appeared with age and was associated with an arthritis-like phenotype (unpublished data). Given these results, we were interested in features of bone marrow from tibia and femur of transgenic mice. Histological examination of limb sections stained with hematoxylin/eosin showed the distinct appearance of adipocytes occupying the marrow cavity (Fig. 5A and Fig. S5A). A clear increase in the number of adipocytes present in the bone marrow of mice was observed upon ageing (Fig. 5A, S5A). Adipocyte densities were significantly higher in DN-Erg compared to wt mice at weeks 6 and weeks 40 (Fig. 5B, 5C). Although adipocytes were readily distinguishable from other cell types present in bone marrow by their morphology (Fig. 5C), Sudan black B staining and immunocytochemistry staining of adiponectin were performed to confirm our observations (Fig. S5B and S5C).
Figure 5. Cytological analysis and adipocyte quantification of femoral bone marrow of wt and DN-Erg mice.
A. Sections of femorotibial joint of week 6- and week 40- wt or DN-Erg mouse. Bars = 1 mm. B. Quantification of adipocytes in bone marrow of DN-Erg and wt femoro-tibial section. The result was expressed as the mean of total adipocyte number per square millimeter of marrow tissue area in the analysed fields of at least three different mouse. C. Cytological examination of bone marrow in a femur section of 40 week-old wt and DN-Erg mice. Left, Magnification: ×2.5. Right: higher magnification view (×10).
Taken together, these histological investigations of bone marrow from adult mice femur revealed that ETS transcription factor family may contribute to the homeostatic balance in skeleton cell plasticity in vivo.
Discussion
Erg expression is associated with early events of cartilage formation during development [21], [24], [31], but its physiological role in this mechanism has been poorly understood. Several specific transcription factors (such as Nkx3.2, Sox9, etc.) have been shown to be involved in the maintenance of the chondrogenic state [32], [33] or in the promotion of transdifferentiation into an adipogenic state [34]. In the present study, we showed that Erg is an important component in the maintenance of the chondrogenic phenotype in vitro, and in the healthy ageing of the skeleton. However, since other ETS family genes are also expressed, to a lesser extent, in cartilage, we cannot rule out that the binding of these transcription factors is also potentially disturbed.
Chondrocytes that are grown in a standard monolayer culture are known to lose their phenotype after a few passages [35]. Furthermore, studies have shown that chondrocytes of the mouse process may transdifferentiate into adipocytes under culture conditions and accumulate lipid droplets [8], [34]. In our study, we observed that chondrocytes isolated from embryos of a transgenic mouse model (expressing a dominant negative form of Erg during cartilage formation and cultured in vitro) and a wt mouse rapidly lose their chondrogenic phenotype. In contrast, only DN-Erg chondrocytes expressed the adipogenic phenotype over time. Significant morphological differences, such as cell morphology and lipid accumulation, were observed in DN-Erg chondrocytes stained in monolayer culture. These observations were confirmed by a comparative transcriptomic analysis of cultured DN-Erg chondrocytes. A high cut-off value was needed to analyse cDNA microarray because of the high number of deregulated genes. GO analysis identified specific groups of regulated gene products associated with a loss of the chondrogenic phenotype and the appearance of adipogenic differentiation markers. Expression of several genes involved in chondrocyte differentiation and hypertrophic maturation dramatically decreased between day 0 and day 20 in DN-Erg compared with wt chondrocytes, whereas the expression of genes involved in matrix metalloproteinases (MMPs) and aggrecanases, leading to articular cartilage destruction, were upregulated. Moreover, the expression of several key features of osteoarthritis pathology [36] and pro-inflammatory mediators (e.g. cysteine proteases cathepsin S and cathepsin C [37]), were detected among the highly regulated genes during DN-Erg chondrocyte culture.
The expression of three master genes Sox9, Runx2 and Pparγ, which are critical for phenotype determination at early stages of mesenchymal cells in cartilage formation, osteogenesis and adipogenesis, respectively, were affected in freshly isolated and in cultured DN-Erg chondrocytes. Knowledge on each transcription factor in the commitment and the maintenance of chondrogenic cell lineages suggests that their deregulation may contribute to the dedifferentiation of chondrocytes and the switch of chondrocytes into adipocytes. Sox9 plays an essential role in the promotion of chondrogenesis [38] and may maintain chondroblasts in an immature state. In addition, the transcription factor Runx2 is a critical enhancer of chondrocyte maturation and osteoblast differentiation. The expression of these two transcription factors was decreased by over 60% in DN-Erg chondrocytes relative to wt chondrocytes. Such low expression levels of key chondrogenesis regulators may explain the early dedifferentiation of cultured DN-Erg chondrocytes. Moreover, as for the depletion of Runx2 in chondrocytes [39], the competition of Erg with a transdominant negative protein favours the loss of the well-established chondrocyte phenotype and the emergence of adipocytes. To show the potent inhibition of the Erg transcription factor in the adipocyte differentiation process, we followed Erg expression in 3T3L1 pre-adipocytes cells (data not shown). The Erg gene is expressed at a very basal level in 3T3L1 cells, and its level did not change when adipocyte differentiation was induced. Moreover Erg overexpression did not affect adipogenenic differentiation of 3T3L1 cells (data not shown). Altogether, these results suggest that although the Erg protein may be involved in the inhibition of the transdifferentiation of chondrocytes into adipocytes, it was not associated with the adipogenic process.
The nuclear receptor Pparγ, mainly involved in the regulation of adipogenesis and in the expression of adipocyte-related differentiation marker genes, also plays an important, albeit complex, role in bone metabolism. On the one hand, Pparγ favours the differentiation of mesenchymal stem cells into adipocytes rather than osteoblasts or chondrocytes [40]; Pparγ overexpression has been reported to promote adipogenic differentiation in growth plate chondrocytes [34], but it has no effect on fully differentiated osteoblasts or osteoclasts [41]. On the other hand, in vivo Pparγ has been shown to be expressed and activated in articular chondrocytes. It is required for endochondral ossification and cartilage development, and has chondroprotective properties against osteoarthritis [42], [43], [44], [45]. Several studies have shown that Pparγ is expressed in hypertrophic chondrocytes. Even if its role remains incompletely resolved, the expression of this transcriptional factor is involved in lipid and energy metabolism [46]. In the present study, Pparγ was expressed in wild-type and DN-Erg chondrocytes, and no difference in the level of Pparγ mRNA expression was found in freshly isolated chondrocytes. However, the expression of Pparγ and its target genes were progressively increased in DN-Erg chondrocytes during culture, which may affect chondrocyte features and accelerate the differentiation pathway to adipocytes.
The present results obtained with primary chondrocytes prompted us to compare skeletal features of transgenic mice with wt mice. It is well known that, with age, bone marrow becomes enriched into adipocytes [47], [48]. Moreover, age-related diseases such as osteoporosis and osteopaenia are described as accompanied by a pronounced accumulation of adipocytes in bone marrow [49], [50], [51], [52]. In contrast with adult wt mice, DN-Erg mice showed predominantly fatty marrow. The function and origin of fatty marrow are still largely unknown [53]. However, these little-studied cells play a more active role than just passively filling the bone cavity [54]. Given the abundant variety of adipokines secreted by adipocytes, the increase in adipocytes in the bone marrow helps to inhibit functions of other bone cells, including osteoblasts and hematopoietic stem cells [55], [56], and structural changes in the surrounding cartilage matrix, thereby disturbing bone regeneration [49], [57], [58], [59]. All these results raise the question of the origin of adipocytes in bone marrow. The bone marrow formation occurs post-natally via the invasion of capillaries through the cortical bone shaft. Downregulation of Sox9 in the hypertrophic zone of the growth plate is required for cartilage-bone transition and bone marrow formation [60]. In this context, our results suggest that DN-Erg transgene expression during chondrogenesis may affect skeletal plasticity and bone quality in adults.
In summary, the present study strongly suggests that the Erg transcription factor is involved in chondrogenesis and plays an important role in the maintenance of the chondrocyte pathway. The potential role of the Ets protein in skeletal cell plasticity was further highlighted by in vivo observations of accelerated formation of fatty marrow in adult DN-Erg mice. Finally, factors affecting cartilage formation may have a high incidence on the occurrence of ageing-related diseases.
Supporting Information
Chondrogenic phenotype was assessed by Alcian blue staining. Chondrocytes were cultured for 15 days, then stained with Alcian blue and observed under a phase-contrast microscope at ×10 magnification.
(TIF)
Oil red O staining of chondrocytes isolated from WT and DN-Erg E18.5 mice and cultured for 20 days. Low-magnification; inset: ×10 magnification.
(TIF)
PCR Analysis of mRNA levels in DN-Erg mice after 0, 6, 10, 15 and 20 days of culture. The reported target gene (i.e. DN-Erg):Hprt transcript ratio at each time was determined par qPCR and was normalised to DN-Erg∶Hprt transcript ratio (set to 1) on day 0. ND = not determined.
(TIF)
PPAR signalling pathway based on Pathway-Express. In the network diagrams, the red boxes indicate genes whose mRNA levels changed during the 20 days of culture.
(TIF)
Histological analysis of bone marrow and Adiponectin detection in femur sections of DN-Erg. A. Cytological examination of bone marrow in femur sections of 6, 24 and 40 week-old wt and DN-Erg mice. Scale bar: 100 µm. B. Lipid staining with Sudan black B (Merck) was used to evaluate lipid droplets within adipocytes according to Soldani et al. [61] on section from femur of 40 week-old DN-Erg mice. Scale bar: 50 µm. C. Adiponectin immunocytochemical staining in bone marrow of 40 week-old DN-Erg mice femur. Scale bar: 50 µm.
(TIF)
List of genes with a 10-fold or more change in expression between day 0 and day 20 of culture in wt chondrocytes in microarray-derived data.
(XLS)
List of genes with a 10-fold or more change in expression between day 0 and day 20 of cultured in DN-Erg chondrocytes in microarray-derived data.
(XLS)
List of genes classified by the “extracellular matrix region”, “metallopeptidase activity”, “cartilage condensation and development”, “ossification” annotations in the Gene Ontology Database.
(XLS)
List of genes classified by the “PPAR signalling” and “adipocyte differentiation” annotations in the Gene Ontology Database.
(XLS)
Acknowledgments
We would like to express our sincere thanks to Antonino Bongiovanni (BioImaging Center Lille – IFR142) and Drs Yvan de Launoit and Bruno Lefebvre (Lille) for their helpful discussions.
Funding Statement
This work was supported by grants from the CNRS, ANR-TecSan (Promocart 2006), La Ligue contre le Cancer (Comité du Pas-de-Calais) and INSERM PRO-A. SF is supported by a fellowship from ANR-TecSan (Promocart 2006). TT is recipient of PhD fellowship from Institut Pasteur de Lille/Région Nord-Pas-de-Calais and Faculté de Médecine Henri Warembourg-Université du Droit et de la Santé Lille 2. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Grigoriadis AE, Heersche JN, Aubin JE (1988) Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol 106: 2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poliard A, Nifuji A, Lamblin D, Plee E, Forest C, et al. (1995) Controlled conversion of an immortalized mesodermal progenitor cell towards osteogenic, chondrogenic, or adipogenic pathways. J Cell Biol 130: 1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 4. Gesta S, Tseng YH, Kahn CR (2007) Developmental origin of fat: tracking obesity to its source. Cell 131: 242–256. [DOI] [PubMed] [Google Scholar]
- 5. Casteilla L, Cousin B, Carmona M (2007) PPARs and Adipose Cell Plasticity. PPAR Res 2007: 68202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Song L, Tuan RS (2004) Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. Faseb J 18: 980–982. [DOI] [PubMed] [Google Scholar]
- 7. Song L, Webb NE, Song Y, Tuan RS (2006) Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells 24: 1707–1718. [DOI] [PubMed] [Google Scholar]
- 8. Heermeier K, Strauss PG, Erfle V, Schmidt J (1994) Adipose differentiation of cartilage in vitro. Differentiation 56: 45–53. [DOI] [PubMed] [Google Scholar]
- 9. Barbero A, Ploegert S, Heberer M, Martin I (2003) Plasticity of clonal populations of dedifferentiated adult human articular chondrocytes. Arthritis Rheum 48: 1315–1325. [DOI] [PubMed] [Google Scholar]
- 10. Ishizeki K, Takahashi N, Nawa T (2002) Phenotypic characteristics of adipocytes generated from Meckel's chondrocytes in response to chick serum in vitro. Cell Tissue Res 309: 251–260. [DOI] [PubMed] [Google Scholar]
- 11. Hall BK, Miyake T (1992) The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat Embryol (Berl) 186: 107–124. [DOI] [PubMed] [Google Scholar]
- 12. de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, et al. (2000) Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol 19: 389–394. [DOI] [PubMed] [Google Scholar]
- 13. Shum L, Nuckolls G (2002) The life cycle of chondrocytes in the developing skeleton. Arthritis Res 4: 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nishimura R, Hata K, Ikeda F, Ichida F, Shimoyama A, et al. (2008) Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J Bone Miner Metab 26: 203–212. [DOI] [PubMed] [Google Scholar]
- 15. Frith J, Genever P (2008) Transcriptional Control of Mesenchymal Stem cell differentiation. Transf Med Hemother 35: 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, et al. (2001) Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A 98: 6698–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Komori T (2002) Runx2, a multifunctional transcription factor in skeletal development. J Cell Biochem 87: 1–8. [DOI] [PubMed] [Google Scholar]
- 18. Provot S, Schipani E (2005) Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun 328: 658–665. [DOI] [PubMed] [Google Scholar]
- 19. Kronenberg HM (2003) Developmental regulation of the growth plate. Nature 423: 332–336. [DOI] [PubMed] [Google Scholar]
- 20. Karsenty G (2008) Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet 9: 183–196. [DOI] [PubMed] [Google Scholar]
- 21. Dhordain P, Dewitte F, Desbiens X, Stehelin D, Duterque-Coquillaud M (1995) Mesodermal expression of the chicken erg gene associated with precartilaginous condensation and cartilage differentiation. Mech Dev 50: 17–28. [DOI] [PubMed] [Google Scholar]
- 22. Ganan Y, Macias D, Duterque-Coquillaud M, Ros MA, Hurle JM (1996) Role of TGF beta s and BMPs as signals controlling the position of the digits and the areas of interdigital cell death in the developing chick limb autopod. Development 122: 2349–2357. [DOI] [PubMed] [Google Scholar]
- 23. Iwamoto M, Tamamura Y, Koyama E, Komori T, Takeshita N, et al. (2007) Transcription factor ERG and joint and articular cartilage formation during mouse limb and spine skeletogenesis. Dev Biol 305: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vlaeminck-Guillem V, Carrere S, Dewitte F, Stehelin D, Desbiens X, et al. (2000) The Ets family member Erg gene is expressed in mesodermal tissues and neural crests at fundamental steps during mouse embryogenesis. Mech Dev 91: 331–335. [DOI] [PubMed] [Google Scholar]
- 25. Sharrocks AD (2001) The ETS-domain transcription factor family. Nat Rev Mol Cell Biol 2: 827–837. [DOI] [PubMed] [Google Scholar]
- 26. Maroulakou IG, Bowe DB (2000) Expression and function of Ets transcription factors in mammalian development: a regulatory network. Oncogene 19: 6432–6442. [DOI] [PubMed] [Google Scholar]
- 27. Karim FD, Urness LD, Thummel CS, Klemsz MJ, McKercher SR, et al. (1990) The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev 4: 1451–1453. [DOI] [PubMed] [Google Scholar]
- 28. Valcourt U, Gouttenoire J, Aubert-Foucher E, Herbage D, Mallein-Gerin F (2003) Alternative splicing of type II procollagen pre-mRNA in chondrocytes is oppositely regulated by BMP-2 and TGF-beta1. FEBS Lett 545: 115–119. [DOI] [PubMed] [Google Scholar]
- 29. Gosset M, Berenbaum F, Thirion S, Jacques C (2008) Primary culture and phenotyping of murine chondrocytes. Nat Protoc 3: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 30. Ueta C, Iwamoto M, Kanatani N, Yoshida C, Liu Y, et al. (2001) Skeletal malformations caused by overexpression of Cbfa1 or its dominant negative form in chondrocytes. J Cell Biol 153: 87–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iwamoto M, Higuchi Y, Koyama E, Enomoto-Iwamoto M, Kurisu K, et al. (2000) Transcription factor ERG variants and functional diversification of chondrocytes during limb long bone development. J Cell Biol 150: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawato Y, Hirao M, Ebina K, Tamai N, Shi K, et al. (2011) Nkx3.2-induced suppression of Runx2 is a crucial mediator of hypoxia-dependent maintenance of chondrocyte phenotypes. Biochem Biophys Res Commun 416: 205–210. [DOI] [PubMed] [Google Scholar]
- 33. Uusitalo H, Salminen H, Vuorio E (2001) Activation of chondrogenesis in response to injury in normal and transgenic mice with cartilage collagen mutations. Osteoarthritis Cartilage 9 Suppl A: S174–179. [PubMed] [Google Scholar]
- 34. Wang L, Shao YY, Ballock RT (2006) Peroxisome Proliferator-Activated Receptor-gamma Promotes Adipogenic Changes in Growth Plate Chondrocytes In Vitro. PPAR Res 2006: 67297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schulze-Tanzil G (2009) Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann Anat 191: 325–338. [DOI] [PubMed] [Google Scholar]
- 36. Wei L, Kanbe K, Lee M, Wei X, Pei M, et al. (2010) Stimulation of chondrocyte hypertrophy by chemokine stromal cell-derived factor 1 in the chondro-osseous junction during endochondral bone formation. Dev Biol 341: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Appleton CT, Pitelka V, Henry J, Beier F (2007) Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum 56: 1854–1868. [DOI] [PubMed] [Google Scholar]
- 38. Akiyama H (2008) Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol 18: 213–219. [DOI] [PubMed] [Google Scholar]
- 39. Enomoto H, Shiojiri S, Hoshi K, Furuichi T, Fukuyama R, et al. (2003) Induction of osteoclast differentiation by Runx2 through receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin regulation and partial rescue of osteoclastogenesis in Runx2−/− mice by RANKL transgene. J Biol Chem 278: 23971–23977. [DOI] [PubMed] [Google Scholar]
- 40. Aubin JE (1998) Advances in the osteoblast lineage. Biochem Cell Biol 76: 899–910. [PubMed] [Google Scholar]
- 41. Akune T, Ohba S, Kamekura S, Yamaguchi M, Chung UI, et al. (2004) PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest 113: 846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bordji K, Grillasca JP, Gouze JN, Magdalou J, Schohn H, et al. (2000) Evidence for the presence of peroxisome proliferator-activated receptor (PPAR) alpha and gamma and retinoid Z receptor in cartilage. PPARgamma activation modulates the effects of interleukin-1beta on rat chondrocytes. J Biol Chem 275: 12243–12250. [DOI] [PubMed] [Google Scholar]
- 43. Hanks BC, Kuroki K, Stoker AM, Cook JL (2010) Evaluation of anti-inflammatory and chondroprotective effects of peroxisome proliferator-activated receptor gamma agonists in cartilage and synovial explants from dogs. Am J Vet Res 71: 1142–1147. [DOI] [PubMed] [Google Scholar]
- 44. Fahmi H, Martel-Pelletier J, Pelletier JP, Kapoor M (2011) Peroxisome proliferator-activated receptor gamma in osteoarthritis. Mod Rheumatol 21: 1–9. [DOI] [PubMed] [Google Scholar]
- 45. Monemdjou R, Vasheghani F, Fahmi H, Perez G, Blati M, et al. (2012) Association of cartilage-specific deletion of peroxisome proliferator-activated receptor gamma with abnormal endochondral ossification and impaired cartilage growth and development in a murine model. Arthritis Rheum 64: 1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stanton LA, Li JR, Beier F (2008) PPARgamma2 expression in growth plate chondrocytes is regulated by p38 and GSK-3. J Cell Mol Med 14: 242–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manolagas SC (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21: 115–137. [DOI] [PubMed] [Google Scholar]
- 48. Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME (2006) Playing with bone and fat. J Cell Biochem 98: 251–266. [DOI] [PubMed] [Google Scholar]
- 49. Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME (1992) Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 102 (Pt 2) 341–351. [DOI] [PubMed] [Google Scholar]
- 50. Koo KH, Dussault R, Kaplan P, Kim R, Ahn IO, et al. (1998) Age-related marrow conversion in the proximal metaphysis of the femur: evaluation with T1-weighted MR imaging. Radiology 206: 745–748. [DOI] [PubMed] [Google Scholar]
- 51. Nuttall ME, Gimble JM (2000) Is there a therapeutic opportunity to either prevent or treat osteopenic disorders by inhibiting marrow adipogenesis? Bone 27: 177–184. [DOI] [PubMed] [Google Scholar]
- 52. Rosen CJ, Bouxsein ML (2006) Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2: 35–43. [DOI] [PubMed] [Google Scholar]
- 53. Majka SM, Barak Y, Klemm DJ (2011) Concise review: adipocyte origins: weighing the possibilities. Stem Cells 29: 1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gevers EF, Loveridge N, Robinson IC (2002) Bone marrow adipocytes: a neglected target tissue for growth hormone. Endocrinology 143: 4065–4073. [DOI] [PubMed] [Google Scholar]
- 55. Corre J, Planat-Benard V, Corberand JX, Penicaud L, Casteilla L, et al. (2004) Human bone marrow adipocytes support complete myeloid and lymphoid differentiation from human CD34 cells. Br J Haematol 127: 344–347. [DOI] [PubMed] [Google Scholar]
- 57. Liu FC, Hung LF, Wu WL, Chang DM, Huang CY, et al. (2010) Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res Ther 12: R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Duque G (2008) Bone and fat connection in aging bone. Curr Opin Rheumatol 20: 429–434. [DOI] [PubMed] [Google Scholar]
- 59. Clabaut A, Delplace S, Chauveau C, Hardouin P, Broux O (2010) Human osteoblasts derived from mesenchymal stem cells express adipogenic markers upon coculture with bone marrow adipocytes. Differentiation 80: 40–45. [DOI] [PubMed] [Google Scholar]
- 60. Hattori T, Muller C, Gebhard S, Bauer E, Pausch F, et al. (2010) SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development 137: 901–911. [DOI] [PubMed] [Google Scholar]
- 61. Soldani G, Losi P, Milioni C, Raffi A (2002) Light microscopy evaluation of polyurethane vascular grafts porosity by Sudan Black B staining. J Microsc 206: 139–145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chondrogenic phenotype was assessed by Alcian blue staining. Chondrocytes were cultured for 15 days, then stained with Alcian blue and observed under a phase-contrast microscope at ×10 magnification.
(TIF)
Oil red O staining of chondrocytes isolated from WT and DN-Erg E18.5 mice and cultured for 20 days. Low-magnification; inset: ×10 magnification.
(TIF)
PCR Analysis of mRNA levels in DN-Erg mice after 0, 6, 10, 15 and 20 days of culture. The reported target gene (i.e. DN-Erg):Hprt transcript ratio at each time was determined par qPCR and was normalised to DN-Erg∶Hprt transcript ratio (set to 1) on day 0. ND = not determined.
(TIF)
PPAR signalling pathway based on Pathway-Express. In the network diagrams, the red boxes indicate genes whose mRNA levels changed during the 20 days of culture.
(TIF)
Histological analysis of bone marrow and Adiponectin detection in femur sections of DN-Erg. A. Cytological examination of bone marrow in femur sections of 6, 24 and 40 week-old wt and DN-Erg mice. Scale bar: 100 µm. B. Lipid staining with Sudan black B (Merck) was used to evaluate lipid droplets within adipocytes according to Soldani et al. [61] on section from femur of 40 week-old DN-Erg mice. Scale bar: 50 µm. C. Adiponectin immunocytochemical staining in bone marrow of 40 week-old DN-Erg mice femur. Scale bar: 50 µm.
(TIF)
List of genes with a 10-fold or more change in expression between day 0 and day 20 of culture in wt chondrocytes in microarray-derived data.
(XLS)
List of genes with a 10-fold or more change in expression between day 0 and day 20 of cultured in DN-Erg chondrocytes in microarray-derived data.
(XLS)
List of genes classified by the “extracellular matrix region”, “metallopeptidase activity”, “cartilage condensation and development”, “ossification” annotations in the Gene Ontology Database.
(XLS)
List of genes classified by the “PPAR signalling” and “adipocyte differentiation” annotations in the Gene Ontology Database.
(XLS)