Abstract
Objective
To assess whether inflammatory markers predict atherosclerotic disease activity after carotid treatment in patients with severe carotid stenosis and nonsignificant coronary artery disease undergoing carotid stenting.
Patients and Methods
From March 1, 2004, to September 30, 2005, a total of 55 consecutive patients (mean ± SD age, 69±8.3 years; 26 men) with severe carotid stenosis and nonsignificant coronary artery disease were treated with carotid stent implantation. Patients were followed up for a period of 5 years for the occurrence of cardiovascular events.
Results
A significant correlation between quantitative analysis of debris entrapped in the filters and inflammatory markers was found. Moreover, the number of particles per filter, the total particles area, and the mean particle axis per filter were significantly higher in patients with clinical events at the follow-up compared with patients without events (87 vs 32, P=.006; 50,118.7 vs 17,782, P=.002; 33.9 vs 30.2, P=.03). At 5-year follow-up we recorded cardiovascular or neurologic events in 11 of the 55 patients (20%). Higher preprocedural levels of high-sensitivity C-reactive protein, interleukin 6 soluble receptor, and interleukin 6 were significantly associated with clinical events at follow-up (P<.001, P=.05, and P=.02, respectively). In particular high-sensitivity C-reactive protein measured at 24 and 48 hours after carotid stenting showed a significant correlation with clinical events (P=.001). Also preprocedural intracellular adhesion molecule 1 and circulating vascular cell adhesion molecule 1 blood concentrations were significantly correlated with a worse prognosis at follow-up (P=.04 and P=.03, respectively).
Conclusion
In patients with severe carotid stenosis and nonsignificant coronary artery disease, inflammation is associated with atherosclerotic disease activity and a worse prognosis. Interleukin 6, interleukin 6 soluble receptor, intracellular adhesion molecule 1, vascular cell adhesion molecule 1, and high-sensitivity C-reactive protein levels at baseline and 24 and 48 hours after carotid stenting are predictive of neurologic and cardiovascular events at follow-up.
Inflammation plays an important role in plaque instability and triggers the progression of atherosclerotic artery disease.1-3 Progressive or unstable plaques put patients at high risk for future clinical events.4-6 In the past 2 decades there has been a growing interest in cardiovascular risk stratification, and preventive therapeutic strategies have been evolved. Several prognostic biomarkers have been evaluated to identify subgroups of patients with progressive atherosclerotic disease who are at high risk for cardiovascular adverse events such as myocardial infarction and stroke.7-11 Coronary and carotid plaque instability have a common inflammatory link, and progression toward myocardial infarction and stroke is predicted by C-reactive protein (CRP) serum levels.8,9,12 In particular, the progression of atherosclerotic plaques in all arterial territories is predicted by high-sensitivity CRP (hs-CRP)13,14 and recurrent instability by its persistent elevated levels.15,16 Inflammatory cell infiltrates were found in coronary and carotid plaques of patients who died of acute coronary syndromes or experienced recent cerebrovascular ischemic events.17-21
Moreover, the role that inflammation plays in atherosclerotic plaque instability may not be confined to one territory alone but may also involve more than one arterial district.22 In fact, Lombardo et al23 demonstrated the presence of widespread arterial inflammatory plaque activation. Therefore, inflammation might be a possible link between coronary and carotid plaque instability and progression.
Therefore, we investigated whether inflammatory markers determined at the time of carotid treatment are predictive of atherosclerotic disease activity and future cardiovascular events defined as cardiac death, myocardial infarction, stroke, transient ischemic attack (TIA), carotid in-stent restenosis, and need for myocardial revascularization in patients with severe carotid stenosis and nonsignificant coronary artery disease undergoing carotid stent implantation.
Patients And Methods
Study Design
We prospectively enrolled 55 consecutive patients with severe carotid stenosis and nonsignificant coronary artery disease who underwent carotid stent implantation from March 1, 2004, until September 30, 2005, at our institution. The study was approved by the institutional review board. The patients included in the study were informed about the procedure and the clinical follow-up and gave their written consent.
Patients eligible for inclusion in the study were 18 years or older; had carotid disease with stenosis of 50% or more in the symptomatic patients or 80% or more in the asymptomatic patients, according to the North American Symptomatic Carotid Endarterectomy Trial method24; and had nonsignificant disease in the coronary tree with stenosis of 50% or less. The confirmation of the presence of carotid stenosis was by means of catheter angiography or both duplex scanning and magnetic resonance angiography (MRA) or computed tomography (CT). Coronary angiography performed as part of the protocol and quantitative computed analysis of stenosis were performed immediately before the carotid stent procedure.
Exclusion criteria were angina pectoris; need for coronary revascularization; coagulopathy; intolerance to heparin, aspirin, or clopidogrel; any cerebrovascular accident in the past 6 weeks; evidence of intraluminal thrombus; vascular disease contraindicating catheterization; intracranial aneurysm exceeding 9 mm in diameter; serum creatinine concentration exceeding 2 mg/dL (to convert serum creatinine to μmol/L, multiply by 88.4); creatinine clearance rate less than 50 mL/min (to convert clearance rate to mL/s, multiply by 0.0167); life expectancy of less than 1 year; or pregnancy.
Carotid Stenting
All the procedures were performed with the patient under local anesthesia using a percutaneous transfemoral approach. Carotid artery stenting was performed using filters as distal protection devices. Heparin (80 IU/kg) was administrated after the introducer sheath had been positioned in the femoral artery. Coronary angiography was performed using a standard technique immediately before the carotid stenting procedure. Then a guiding catheter was positioned proximal to the bifurcation in the common carotid artery. To reduce the amount of angiographic dye administered during the stent implantation procedure, a “road-mapping” modality of acquisition was performed. Predilation of the lesion was performed when necessary after protection device placement. After implantation, stents were postdilated to achieve a residual stenosis of 30% or less.
Medication
Aspirin, 100 mg/d, and clopidogrel, 75 mg/d, were administered 7 days before carotid artery stenting. Clopidogrel was prescribed for 1 month, and then treatment was stopped; aspirin was used indefinitely. Patients received 40 mg of atorvastatin once a day for 1 month, independent of cholesterol levels. Then the dosage of this drug was adjusted to maintain low-density lipoprotein cholesterol levels of 80 mg/dL or less (to convert low-density lipoprotein cholesterol to mmol/L, multiply by 0.0259).
Follow-up
In all patients clinical evaluation was performed at 48 hours, 30 days, and 6 months after treatment and every year for 5 years. A complete neurologic examination, including the National Institutes of Health Stroke Scale, was performed by a board-certified neurologist before and after the procedure. Color duplex ultrasonography evaluation and MRA or CT were performed before hospital discharge, at 6 months, and every year for the entire follow-up period. In-stent restenosis (≥50%) detected by duplex ultrasonography was defined according to the combination of a peak systolic velocity value of 225 cm/s or greater and an internal carotid artery to common carotid artery ratio of 2.5 or higher.24,25
The occurrence of any neurologic event was carefully evaluated during hospitalization and follow-up using CT or MRA. An independent neurologist masked to clinical data performed the assessments. A TIA was defined as a focal neurologic deficit lasting less than 24 hours. A stroke was considered disabling (major) if the patient had a Rankin score equal to or greater than 3 at 90 days. The primary end point was the correlation between inflammatory markers and magnitude of debris entrapped in the filter used during carotid artery stenting procedures. The secondary end point was the event-free survival at 5 years (defined as freedom from cardiac death, myocardial infarction, stroke, TIA, carotid in-stent restenosis, and need for myocardial revascularization).
Histopathologic Evaluation
To perform the histomorphometric analysis of plaque debris, each filter was examined with a stereomicroscope, digitalized, fixed in 10% neutral buffered formalin, and sent to the histology core laboratory for cytologic analysis. The pathologist was masked to serum marker results.
The morphometric analysis was performed using the Image-Pro Plus software (Media Cybernetics, Bethesda, MD), and the total area of the filter membrane and the area covered by particulate material were quantified. Moreover, at 100-fold magnification the following parameters were also counted: number of particles per filter, area of each particle, total particles area per filter, and major and minor axes of a single particle.
The material removed from the filters was stained with hematoxylin-eosin for the histologic study. A particle was identified as a thrombus fragment if it was composed of a conglomerate of erythrocytes and embedded strands of fibrin and as plaque fragment if it also contained cholesterol clefts surrounded by some foam cells.
Measurement of Serum Inflammatory Markers
Antecubital venous samples were obtained on admission to the hospital for measurement of all inflammatory markers and 24 and 48 hours after the procedure for hs-CRP determination. Then, blood was centrifuged at 3500g for 5 minutes and stored at −80°C.
Measurement included hs-CRP values, pregnancy-associated protein A, interleukin (IL) 1, IL-6, IL-6 soluble receptor (IL-6-SR), soluble CD40, IL-4, IL-10, soluble tumor necrosis factor, intracellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1). For hs-CRP measurement, a high-sensitivity assay (N Latex CRP MONO; DADE Behring, Deerfield, IL) with a detection level of 0.03 mg/dL (to convert hs-CRP to mg/L, multiply by 10) and coefficient of variation of 4.6% was used.
Serum IL-6, IL-10, and IL-4 levels were measured by enzyme-linked immunosorbent assay (Diaclone; Gen-Probe Inc, San Diego, CA). The detectable limits for IL-6, IL-10, and IL-4 were 0.8, 1.30, and 0.31 pg/mL. IL-8, IL-1β, IL-6-SR, soluble VCAM, soluble ICAM, soluble CD40L (Diaclone), and soluble CD40 (IBL, Minneapolis, MN) were measured by enzyme-linked immunosorbent assay (Immunotech, Prague, Czech Republic).
Statistical Analyses
A dedicated file was used to collect all clinical and procedural data. Analyses of in-hospital and follow-up data were possible for all patients enrolled in the study. Continuous variables are presented as mean ± SD and are compared using the 2-sided t test for unpaired data or analysis of variance, as appropriate. Because distribution of all inflammatory marker levels appeared to be left skewed, these markers are normalized by log transformation and expressed as median (interquartile range). Categorical variables are expressed as proportions and percentages and are compared with the χ2 test. Linear regression analysis was performed to examine correlation between histologic analysis of debris removed from the filters after carotid stenting procedure and inflammatory markers. Logistic regression analyses were performed to examine the association between symptoms and inflammatory markers. Analysis of variance was performed to evaluate increment of hs-CRP at baseline, 24 hours, and 48 hours. The level of statistical significance for hypothesis testing was P<.05. All statistical analyses were performed with Statview 5.0 for Windows 8.0 (SAS Institute Inc, Cary, NC).
Results
A total of 55 consecutive patients with complete baseline and follow-up data were included in the final analysis. The median age was 69 years (interquartile range, 60-80 years), and 26 of the 55 patients (47%) were male. Demographic data and clinical characteristics of the study sample are presented in Table 1. In particular, 1 patient (2%) had had previous myocardial infarction and 27 (49%) had a history of stroke or TIA. In 11 patients (20%), a history of stroke was found without residual or recurrent symptoms; in these patients, the median interval between prior stroke and inclusion in the study was 3.9 years (range, 1.9-11.0 years). All patients successfully underwent the carotid stent procedure as indicated in Table 2.
TABLE 1.
| Characteristic | Patients without events (n=44) | Patients with events (n=11) | P value |
|---|---|---|---|
| Age (y), mean ± SD | 71.0±8.4 | 68.1±6.9 | .29 |
| Male | 20 (45) | 6 (55) | .59 |
| BMI, mean (IQR) | 27.3 (25.6-28.8) | 27.1 (24.5-30.0) | .47 |
| Family history of CAD | 13 (30) | 3 (27) | .88 |
| Hypertension | 36 (82) | 11 (100) | .13 |
| Diabetes mellitus | 18 (41) | 5 (45) | .78 |
| Current smoking | 10 (23) | 1 (9) | .31 |
| Dyslipidemia | 29 (66) | 10 (90) | .14 |
| Total cholesterol (mg/dL), mean (IQR) | 187 (156-209) | 187 (150.5-220) | .59 |
| LDL-C (mg/dL), mean (IQR) | 100.5 (81-135) | 96.5 (56-136) | .66 |
| HDL-C (mg/dL), mean (IQR) | 50 (43-60) | 47 (41-57) | .91 |
| Chronic renal failurec | 2 (5) | 1 (9) | .80 |
| Preprocedural creatinine (mg/dL), mean (IQR) | 1.08 (0.9-1.3) | 1.1 (1.0-1.2) | .81 |
| Degree of stenosis in treated carotid lesion, mean ± SD | 78.5±8.7 | 79.4±11.8 | .76 |
| Bilateral carotid stenosis | 15 (34) | 4 (36) | .89 |
| Previous myocardial infarction | 1 (2) | 0 | .61 |
| History of stroke or TIA | 23 (52) | 4 (36) | .35 |
| Left ventricle ejection fraction, mean ± SD | 55.7±6.8 | 54.7±11.2 | .19 |
| Preoperative statin treatment | 30 (68) | 8 (72) | .77 |
Data are presented as No. (percentage) unless indicated otherwise. BMI = body mass index; CAD = coronary artery disease; HDL-C = high-density lipoprotein cholesterol; IQR = interquartile range; LDL-C = low-density lipoprotein cholesterol; TIA = transient ischemic attack.
SI conversion factors: To convert total cholesterol to mmol/L, multiply by 0.0259; to convert LDL-C to mmol/L, multiply by 0.0259; to convert HDL-C to mmol/L, multiply by 0.0259; to convert serum creatinine to μmol/L, multiply by 88.4.
Serum creatinine concentration exceeding 2 mg/dL and creatinine clearance rate of less than 50 mL/min.
TABLE 2.
Characteristics of the Carotid Stenting Procedure
| Characteristic | Patients without events (n=44) | Patients with events (n=11) | P value |
|---|---|---|---|
| Length of lesion (mm), mean ± SD | 14.3±5.4 | 15.2±6.4 | .31 |
| Predilation of stenosis | 13 (30) | 3 (27) | .88 |
| Duration of procedure (min), mean (IQR) | 30 (15-98) | 27 (14-92) | .28 |
| Volume of angiographic dye administered (mL), mean ± SD | 44.8±24.3 | 48±26.4 | .23 |
| Type of stent used | |||
| Carotid Wallstent (Boston Scientific, Natwick, MA) | 31 (70) | 5 (45) | .17 |
| Acculink (Abbott, Abbott Park, IL) | 13 (30) | 6 (55) | .82 |
| Cerebral protection used | |||
| FilterWire EZ (Boston Scientific) | 39 (89) | 8 (73) | .24 |
| Accunet (Abbott) | 5 (11) | 3 (27) | .18 |
Data are presented as median (interquartile range).
At the 5-year follow-up, 11 patients (20%) experienced neurologic or cardiovascular events; in particular, 1 patient (2%) died (cardiac death), 2 patients (4%) had myocardial infarction or stroke, 6 patients (11%) had progression of coronary artery disease followed by myocardial revascularization, and 2 patients (4%) had carotid stent restenosis (Table 3).
TABLE 3.
Clinical Events in 55 Patients Who Underwent Carotid Stenting at 5-Year Follow-up
| Event | No. (%) of events |
|---|---|
| Myocardial infarction | 1 (2) |
| Stroke | 1 (2) |
| Carotid in-stent restenosis | 2 (4) |
| Coronary artery bypass grafting | 1 (2) |
| Percutaneous coronary angioplasty | 5 (9) |
| Death (cardiac) | 1 (2) |
The debris entrapped in the filters was composed of acellular material rich in the cholesterol cleft, necrotic material, calcium precipitates, fibrin, platelets, collagen fibers, and unidentified fibrillate. No specific component related to the capture of larger particles was observed. However, we found a significant correlation between the magnitude of debris entrapped in the filters and inflammatory markers (Figure 1 and Figure 2). Interestingly, the number of particles per filter, the total particles area, and the mean particle axis per filter were significantly higher in patients with cardiovascular and neurologic events at the follow-up (Table 4).
FIGURE 1.
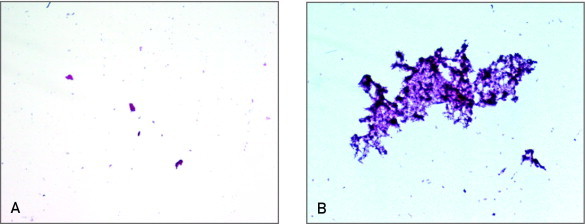
Histologic analysis of debris removed from the filters after carotid stenting. A, Low number of debris entrapped in the filter in a patient with low levels of inflammatory markers before carotid stent implantation. B, Elevated number of particles in a patient with high levels of inflammatory markers before the procedure. The debris entrapped in the filter was composed of acellular material rich in cholesterol cleft, necrotic material, and calcium precipitates.
FIGURE 2.
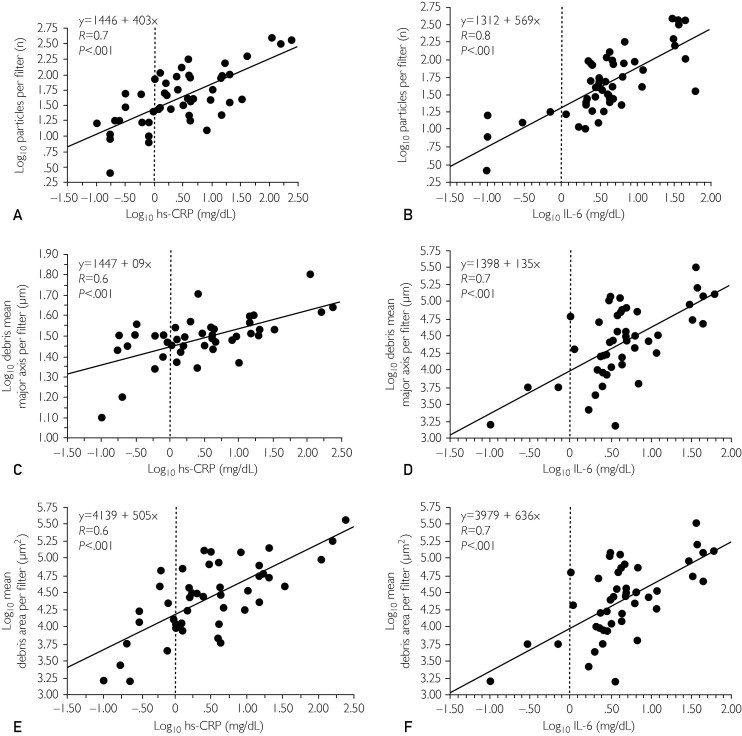
Correlation between inflammatory markers and debris removed from the filters. Correlation between high-sensitivity C-reactive protein (hs-CRP) (left) and interleukin 6 (IL-6) (right) preprocedural values and histologic analysis of debris removed from the filters after carotid stenting procedure: number of particles (A, B), mean major axis per filter (C, D), and mean debris area per filter (E, F).
TABLE 4.
Correlation Among Histologic Parameters, Neurologic Events, and Cardiovascular Events
| Histologic parameter | Patients without events (n=44) | Patients with events (n=11) | P value |
|---|---|---|---|
| Particles per filter | 32 (18-75) | 87 (43-262) | .006 |
| Mean particle major axis per filter | 30.2 (26.8-31.8) | 33.9 (31.8-40.6) | .03 |
| Total particles area per filter | 17,782.8 (6138.0-40,036.8) | 50,118.7 (29,043.6-120,226.4) | .002 |
Data are presented as median (interquartile range).
The preprocedural levels of the inflammatory markers hs-CRP, IL-6-SR, and IL-6 were significantly associated with clinical events at 5-year follow-up (P<.001, P=.05, and P=.02, respectively). In these patients a significant increase was observed in hs-CRP values at 24 and 48 hours after carotid stent implantation compared with patients with normal preprocedural values (P=.03) (Figure 3A). In particular, hs-CRP measured at 24 and 48 hours after carotid stent implantation showed at each time a significant correlation with clinical events (P=.001) (Figure 3B), and more interestingly we found that an hs-CRP level lower than 0.8 mg/dL at 48 hours after the procedure is associated with the absence of events at follow-up. In addition, preprocedural ICAM-1 and VCAM-1 blood concentrations were significantly correlated with a worse prognosis at 5-year follow-up (P=.04 and P=.03, respectively) (Table 5).
FIGURE 3.
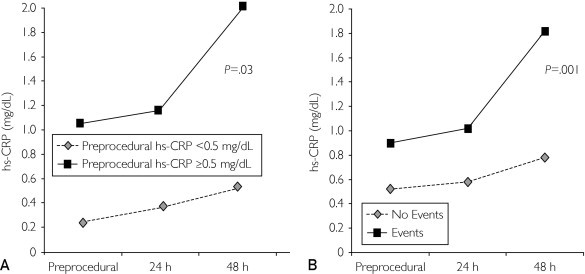
A, Inflammatory response after stent implantation. The inflammatory response was enhanced by stent implantation in patients with preprocedural levels of high-sensitivity C-reactive protein (hs-CRP) greater than or equal to 0.5 mg/dL compared with patients with hs-CRP levels less than 0.5 mg/dL (P=.03). B, hs-CRP values according to cardiovascular events at 5-year follow-up. A significant correlation was noted between hs-CRP values at baseline and at 24 and 48 hours after the procedure and clinical events at 5-year-follow-up (P=.001). To convert hs-CRP levels to mg/L, multiply by 10.
TABLE 5.
| Inflammatory markers | Patients without events (n=44) | Patients with events (n=11) | P value |
|---|---|---|---|
| hs-CRP (mg/dL) | 0.2 (0.1-0.4) | 1.3 (0.4-2) | .0008 |
| PAPP-A (μg/mL) | 49.2 (27.8-69.5) | 30.3 (21.1-66.7) | .21 |
| Interleukin 1 (pg/mL) | 10.6 (2.8-22.2) | 4.7 (0.2-7.3) | .50 |
| Interleukin 6 (pg/mL) | 3.4 (2.0-4.9) | 6.5 (3.2-36.9) | .02 |
| Interleukin 8 (pg/mL) | 24.1 (3.9-43.7) | 10.5 (6.8-61.9) | .92 |
| Soluble interleukin 6 receptor (ng/mL) | 1865.0 (1482.5-2422.2) | 2411.9 (1996.5-2738.2) | .05 |
| Soluble CD40 (pg/mL) | 51.7 (39.2-65.8) | 42.6 (33.8-66.4) | .62 |
| Interleukin 4 (pg/mL) | 0.22 (0.1-0.6) | 0.4 (0.1-0.4) | .87 |
| Interleukin 10 (pg/mL) | 0 (0-0.4) | 0 (0-3.4) | .21 |
| Soluble tumor necrosis factor (ng/mL) | 2.1 (1.6-3.6) | 3.1 (1.6-3.6) | .84 |
| ICAM-1 (ng/mL) | 450.0 (330.0-560.0) | 600.0 (437.5-930.0) | .04 |
| VCAM-1 (ng/mL) | 862.5 (705.0-995.0) | 975.0 (770.0-1575.0) | .03 |
Data are presented as median (interquartile range). hs-CRP = high-sensitivity C-reactive protein; ICAM-1 = intracellular adhesion molecule1; PAPP-A = pregnancy-associated protein A; VCAM-1 = vascular cell adhesion molecule 1.
SI conversion factors: To convert hs-CRP levels to mg/L, multiply by 10.
Discussion
In our prospective study, we found that inflammatory markers are correlated with disease activity demonstrated by the amount of plaque fragments caught in the filters, and we confirmed a correlation between inflammation and subsequent events in patients with severe carotid stenosis undergoing carotid stent implantation. In these patients, increasing levels of the inflammatory biomarkers, such as hs-CRP, IL-6-SR, IL-6, ICAM-1, and VCAM-1 blood concentrations at baseline and immediately after intervention, are predictive of future clinical events. In particular, hs-CRP measured at 24 and 48 hours after the procedure showed at each time a significant correlation with a worse prognosis at the 5-year follow-up, which is consistent with our previous observation after coronary artery stent implantation.26 More interesting is the observation of the link between inflammation and events at follow-up, including cardiovascular events in patients with stable nonsignificant coronary artery disease at the moment of carotid arteries procedure. This observation underlines the link between inflammation and progression of disease also in remote territories such as coronary arteries. The presence of unstable plaques is associated with a markedly increased risk for clinical complications in virtually any arterial district.3,27 These vulnerable lesions are histologically heterogeneous and may present a thin fibrous cap of the atheroma, with superficial erosions, thrombus apposition, fibrocalcified nodules, an inflammatory necrotic lipid core, and intraplaque hemorrhage.27 Interestingly, in our study we found a strong correlation between periprocedural inflammatory markers and the magnitude of plaque debris in the filters. The amount of histologic particles might be due to the disruption during stent implantation procedures of more vulnerable plaques, demonstrating in such patients the presence of active plaques. In these patients we might speculate on the possibility of a link between the magnitude of debris in the filters and disease activity. This is in line with the recent observation by Moustafa et al28 that embolic events distal to carotid stenosis are related to plaque inflammation detected by fluorodeoxyglucose positron emission tomography.
Acute-phase reactants are markers of the cytokine-dependent inflammatory activation in the arterial wall. This activation is a signal of the inflammatory activity surrounding the necrotic lipid core.29 However, hs-CRP, IL-6-SR, IL-6, ICAM, and VCAM not only are markers of atherosclerosis risk but also directly promote atherosclerosis progression.30-32
Whether high levels of inflammatory biomarkers are due to the presence of a single vulnerable lesion or identify a “vulnerable patient” with several coexisting high-risk lesions in different arterial territories is yet to be confirmed. Although CRP is synthesized by the liver in response to an inflammatory trigger,32 it has also been found in atherosclerotic plaque.33 Yasojima et al34 have suggested that arterial wall cells can synthesize CRP. Ishikawa et al35 identified a CRP immunoreaction and showed that CRP messenger RNA is expressed in vulnerable plaques, suggesting that CRP is synthesized locally in the plaques. More recently, Inoue et al36 demonstrated a translesional gradient of CRP, which provided evidence of the local CRP production in coronary atherosclerotic plaques. This finding suggests that the magnitude of the translesional CRP gradient may represent an important marker of a vulnerable plaque. In addition, this study demonstrated that after percutaneous coronary intervention, the transcardiac gradient of CRP increases, suggesting that vessel wall injury during percutaneous coronary intervention is potentially responsible for the increased CRP production. Moreover, as we demonstrated in a previous study,37 systemic inflammatory activation is probably due to the presence of activated inflammatory cells in unstable atherosclerotic plaque. In these patients, the stent is a powerful trigger that amplifies the inflammatory response. This local inflammatory response was enhanced by stent implantation in patients with high preprocedural levels of CRP compared with patients with normal preprocedural levels of CRP, with a significantly higher proportion of patients with adverse events at the 12-month follow-up.
For the first time, to our knowledge, we demonstrate that elevated levels of inflammatory biomarkers are also predictive of future events also in remote regions, identifying a “vulnerable patient” with several coexisting high-risk lesions in different arterial segments. Elevation of hs-CRP, IL-6, IL-6-SR, ICAM-1, and VCAM-1 concentrations indicates enhanced inflammation and may help to identify those patients who are generally susceptible to clinical events. The possibility of identifying these patients who are at risk for future clinical events in remote regions is potentially relevant, and consistent measurement of inflammatory biomarkers may represent an important implement for new therapeutic strategies.
Several limitations of the study have to be acknowledged. First, the study population was relatively small, and the possibility of selection bias cannot be excluded, although consecutive patients who were eligible and consented to participate in the study were selected. However, we consider this a pilot study, in which we demonstrated a possible link between systemic inflammation and active carotid disease. Larger studies will be needed to confirm whether inflammation can predict cardiovascular adverse events.
Acute-phase parameters, progression of atherosclerotic disease, or the occurrence of clinical adverse events can be affected by several medications; residual confounding by the changes of the medication during the study cannot be excluded. However, all patients received the same dosage of atorvastatin for 1 month; then the dosage of this drug was adjusted to maintain low-density lipoprotein cholesterol levels of 80 mg/dL or less. Regarding this strategy, adherence to therapy and the follow-up of lipid level monitoring in patients with and without events was similar.
Finally, one other limitation is the lack of evaluation for the presence of atherosclerotic disease in other territories (ie, peripheral arteries disease). Therefore, in our study it is not possible to speculate between the systemic atherosclerotic burden and the magnitude of inflammation.
Conclusion
Inflammatory markers are associated with the presence of active disease demonstrated by the amount of plaque fragments caught in the filters during stent implantation. In patients with carotid artery disease undergoing stenting, the magnitude and persistence of inflammation are associated with the progression of atherosclerotic disease and worse prognosis. Elevated levels of IL-6, IL-6-SR, ICAM-1, VCAM-1, and hs-CRP measured before and at 24 and 48 hours after carotid stenting are also predictive of future cardiovascular events also in remote regions and can identify a “vulnerable patient.”
Acknowledgments
We acknowledge the Centro per la Lotta contro l'Infarto – Onlus Foundation and Rome Heart Research for the skillful assistance with image acquisition and data evaluation.
Footnotes
Grant Support: This study was supported in part by a Fondo per gli Investimenti della Ricerca di Base grant.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Lusis A.J. Atherosclerosis. Nature. 2000;407(6801):223–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naghavi M., Libby P., Falk E. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108(4):1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 4.Kolodgie F.D., Gold H.K., Burke A.P. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349(24):2285–2287. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 5.Naghavi M., Libby P., Falk E. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part II. Circulation. 2003;108(15):1172–1178. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- 6.Libby P., Ridker P.M., Hansson G.K. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridker P.M., Rifai N., Rose L., Buring J.E., Cook N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of the first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 8.Ridker P.M., Cushman M., Stampfer M.J., Tracy R.P., Hennekens C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336(14):937–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 9.Koenig W., Sund M., Frolich M. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study. 1984 to 1992. Circulation. 1999;99(2):237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 10.Rost N.S., Wolf P.A., Kase C.S. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attacks: the Framingham Study. Stroke. 2001;32(11):2575–2579. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- 11.Ridker P.M., Hennekens C.H., Buring J.E., Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 12.Devaraj S., Xu D.Y., Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107(3):398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 13.Verma S., Li S.H., Badiwala M.V. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105(16):1980–1986. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- 14.Schillinger M., Exner M., Amighi J. Joint effects of C-reactive protein and glycated haemoglobin in predicting future cardiovascular events in patients with advanced atherosclerosis. Circulation. 2003;108(19):2323–2328. doi: 10.1161/01.CIR.0000095267.24234.00. [DOI] [PubMed] [Google Scholar]
- 15.Verma S., Wang C.H., Li S.H. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 16.Gaspardone A., Crea F., Versaci F. Predictive value of C-reactive protein after successful coronary-artery stenting in patients with stable angina. Am J Cardiol. 1998;82(4):515–518. doi: 10.1016/s0002-9149(98)00370-1. [DOI] [PubMed] [Google Scholar]
- 17.Mauriello A., Sangiorgi G., Fratoni S. Diffuse and active inflammation occurs in both vulnerable and stable plaque of entire coronary tree: a histopathological study of patients dying of acute myocardial infarction. J Am Coll Cardiol. 2005;45(10):1585–1593. doi: 10.1016/j.jacc.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 18.Golledge J., Greenhalgh R.M., Davies A.H. The symptomatic carotid plaque. Stroke. 2000;31(3):774–781. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]
- 19.Kolodgie F.D., Nakazawa G., Sangiorgi G., Ladich E., Burke A.P., Virmani R. Pathology of atherosclerosis and stenting. Neuroimaging Clin N Am. 2007;17(3):285–301. doi: 10.1016/j.nic.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jander S., Sitzer M., Schumann R. Inflammation in high-grade carotid stenosis: a possible role for macrophages and T cells in plaque destabilization. Stroke. 1998;29(8):1625–1630. doi: 10.1161/01.str.29.8.1625. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein J.A. Angiographic plaque complexity: the tip of the unstable plaque iceberg. J Am Coll Cardiol. 2002;39(9):1446–1467. doi: 10.1016/s0735-1097(02)01772-2. [DOI] [PubMed] [Google Scholar]
- 22.Buffon A., Biasucci L.M., Liuzzo G., D'Onofrio G., Crea F., Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med. 2002;347(1):5–12. doi: 10.1056/NEJMoa012295. [DOI] [PubMed] [Google Scholar]
- 23.Lombardo A., Biasiucci L.M., Lanza G.A. Inflammation as a possible link between coronary and carotid plaque instability. Circulation. 2004;109(25):3158–3163. doi: 10.1161/01.CIR.0000130786.28008.56. [DOI] [PubMed] [Google Scholar]
- 24.Stanziale S.F., Wholey M.H., Boules T.N., Selzer F., Makaroun M.S. Determining in-stent stenosis of carotid arteries by duplex ultrasound criteria. J Endovasc Ther. 2005;12(3):346–353. doi: 10.1583/04-1527.1. [DOI] [PubMed] [Google Scholar]
- 25.Chi Y.W., White C.J., Woods T.C., Goldman C.K. Ultrasound velocity criteria for carotid in-stent restenosis. Catheter Cardiovasc Interv. 2007;69(3):349–354. doi: 10.1002/ccd.21032. [DOI] [PubMed] [Google Scholar]
- 26.Gaspardone A., Crea F., Versaci F. Predictive value of C-reactive protein after successful coronary-artery stenting in patients with stable angina. Am J Cardiol. 1998;82(4):515–518. doi: 10.1016/s0002-9149(98)00370-1. [DOI] [PubMed] [Google Scholar]
- 27.Kolodgie F.D., Gold H.K., Burke A.P. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349(24):2285–2287. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 28.Moustafa R.R., Izquierdo-Garcia D., Fryer T.D. Carotid plaque inflammation is associated with cerebral microembolism in patients with recent transient ischemic attack or stroke: a pilot study. Circ Cardiovac Imaging. 2010;3(5):536–541. doi: 10.1161/CIRCIMAGING.110.938225. [DOI] [PubMed] [Google Scholar]
- 29.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 30.Pasceri V., Willerson J.T., Yeh E.T. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 31.Verna S., Wang C.H., Li S.H. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106(8):913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 32.Castel J.V., Gomez-Lechon M.J., Fabra R., Trullenque R., Heinrich P.C. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12(5):1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- 33.Ishikawa T., Hatakeyama K., Imamura T. Involvement of C-reactive protein obtained by directional coronary atherectomy in plaque instability and developing restenosis in patients with stable or unstable angina pectoris. Am J Cardiol. 2003;91(3):287–292. doi: 10.1016/s0002-9149(02)03156-9. [DOI] [PubMed] [Google Scholar]
- 34.Yasojima K., Schwab C., McGeer E.G., McGeer P.L. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001;158(3):1039–1051. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishikawa T., Hatakeyama K., Imamura T. Increased adrenomedullin immunoreactivity and mRNA expression in coronary plaques obtained from patients with unstable angina. Heart. 2004;90(10):1206–1210. doi: 10.1136/hrt.2003.017921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue T., Kato T., Uchida T. Local release of C-reactive protein from vulnerable plaque or coronary arterial wall injured by stenting. J Am Coll Cardiol. 2005;46(2):239–245. doi: 10.1016/j.jacc.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 37.Versaci F., Gaspardone A., Tomai F., Crea F., Chiariello L., Gioffrè P.A. Predictive value of C-reactive protein in patients with unstable angina pectoris undergoing coronary artery stent implantation. Am J Cardiol. 2000;85(1):92–95. doi: 10.1016/s0002-9149(99)00612-8. A8. [DOI] [PubMed] [Google Scholar]


