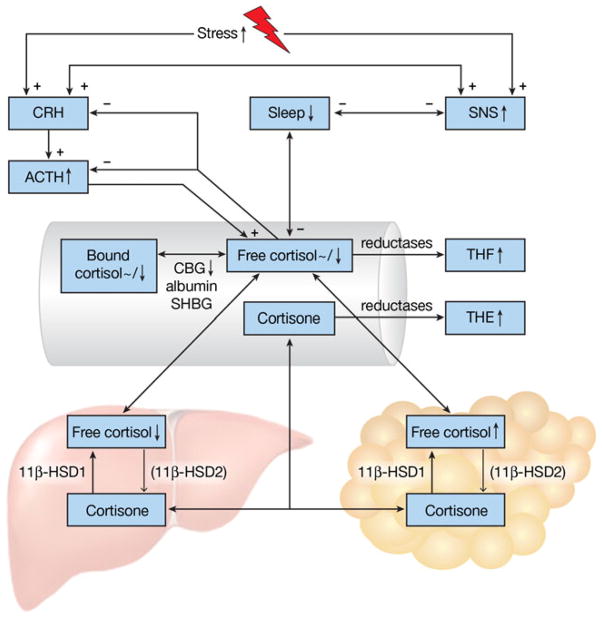
Fig. 1.
Schematic representation of HPA-axis and sleep alterations in obesity. Plus and minus signs indicate positive and negative effects, respectively. Upward/downward arrows and tildes (~) depict increased/decreased and unaltered levels, respectively, of depicted phenomena in obese individuals. Obese subjects often experience an increased stress exposure, leading to increased activity of the sympathetic nervous system (SNS) and release of corticotrophin-releasing hormone (CRH). CRH triggers release of adrenocorticotropic hormone (ACTH), which levels are elevated in obesity. ACTH stimulates cortisol release into the bloodstream. Cortisol, in turn, inhibits CRH and ACTH release. The HPA-axis and the SNS stimulate each other in a reverberating circuit and are able to affect sleep. Inadequate sleep is more common in obese subjects. Circulating cortisol levels, both in free form or bound to corticosteroid binding globulin (CBG), albumin or sex hormone binding globulin (SHBG), are reported unaltered of decreased in obesity. In tissues, cortisol is converted to inactive cortisone and vice versa by 11β hydroxysteroid dehydroge-nase type 2 11β-HSD2 and 11β-HSD1, respectively. 11β-HSD1 is mostly present in the liver and adipose tissue. 11β-HSD2 is present in smaller quantities in these tissues, as depicted by the thinner arrow, whereas it is abundantly expressed in the kidney and colon. Hepatic cortisol levels are decreased in obese subjects, due to decreased 11β-HSD1 levels. In contrast, cortisol levels in adipose tissue are increased due to increased levels of 11β-HSD1. Cortisol and cortisone are metabolized by reductases to the inactive tetrahydrometabolites (THM) of tetrahydrocortisol (THF) and tetrahydrocortisone (THE), respectively. Urinary levels of THM and THE are usually increased in obese subjects