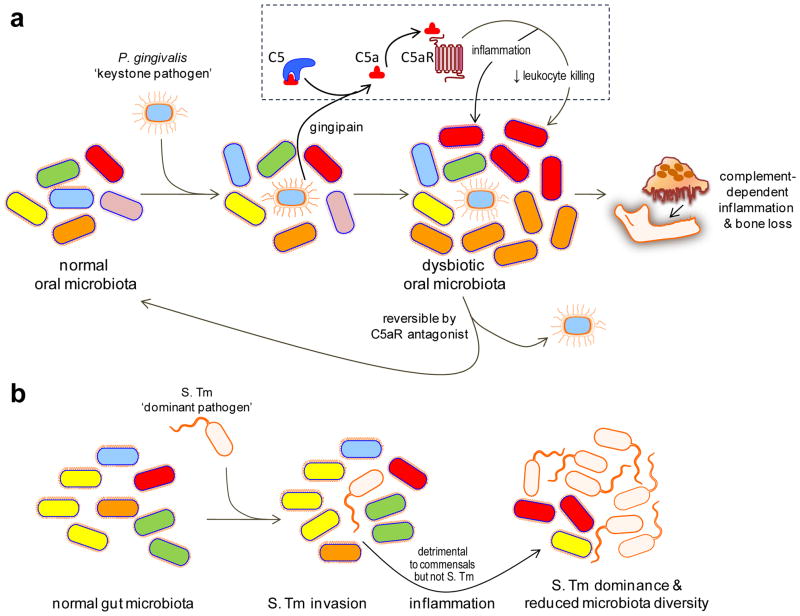
Figure 1. Keystone vs. dominant pathogens.
(a) Keystone pathogen-induced dysbiotic disease. Despite its low-level colonization of the periodontium, P. gingivalis causes inflammatory periodontitis through dysbiosis, i.e., an unbalancing of the relative abundance of individual components of the microbiota compared with their abundancies in health. This activity requires the bacterium’s gingipain, a C5 convertase-like enzyme which cleaves C5 generating high levels of C5a locally. C5a-induced activation of C5aR triggers inflammation but is also critically involved in a subversive crosstalk (with TLR2) that impairs leukocyte killing. The ability of P. gingivalis to orchestrate inflammatory disease via community-wide effects, while being a minor constituent of this community, qualifies it as a keystone pathogen. This process is reversible since C5aR blockade promotes the clearance of P. gingivalis and negates its dysbiotic effects. (b) Dominant pathogen-induced inflammation and effects on the microbiota. Salmonella enterica Serovar Typhimurium (S. Tm) induces and exploits inflammation to alter the composition of and outgrow the indigenous gut microbiota leading to colitis. Therefore, S. Tm incites inflammatory disease while becoming the dominant species, in stark contrast to P. gingivalis which acts as a “keystone” that supports the oral microbiota. Adapted from ref. 78.