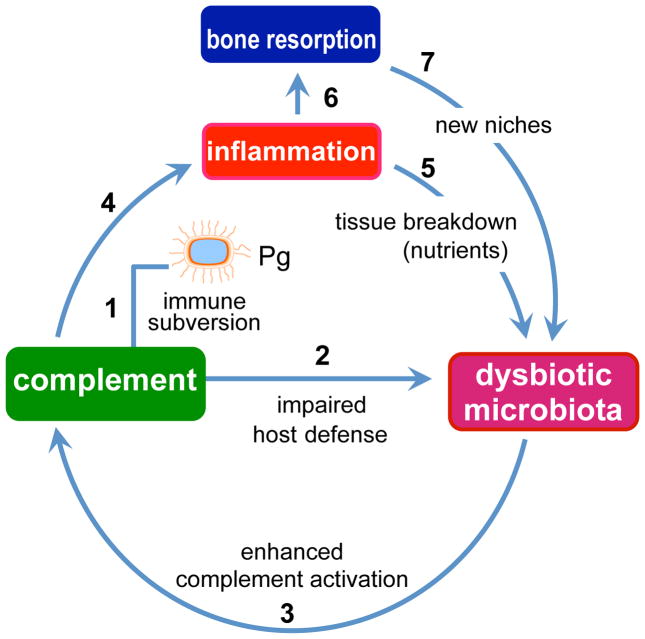
Figure 2. P. gingivalis-induced dysbiosis and periodontal disease.
P. gingivalis subverts complement and impairs host defense leading to overgrowth of oral commensal bacteria, which cause complement-dependent inflammation. Inflammatory tissue destruction is favorable to further bacterial growth as it provides a nutrient-rich gingival inflammatory exudate (degraded host proteins and hemin, a source of essential iron). These environmental changes are better exploited by and thus favor proteolytic and asaccharolytic bacteria, leading to compositional changes in the bacterial community. Inflammatory bone resorption, moreover, provides the dysbiotic microbiota with new niches for colonization. These alterations collectively lead to and sustain periodontal disease. The numbers indicate a possible sequence of events, which set off a self-feeding “vicious” cycle.