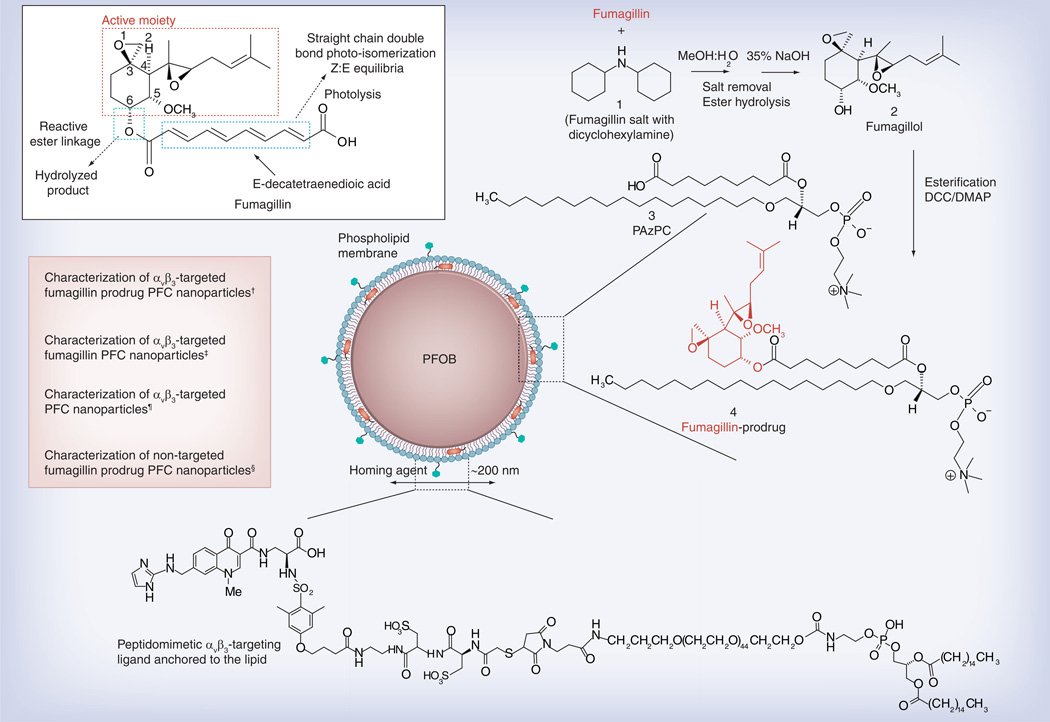
Figure 1. Structure and active sites of fumagillin with their sources of instability indicated (see figure on previous page).
Synthetic strategy for the preparation of Sn-2 fumagillin prodrug and development of site-targeted nanoparticles: saponification of fumagillin with MeOH:water (1:1), 35% NaOH; esterification with PAzPC, DCC/DMAP; preparation of a lipid-thin film from a phospholipid mixture of 98.7 mole% lecithin phosphatidylcholine, 0.15 mole% of αvβ3-ligand-conjugated lipid and 1.12 mole% of fumagillin prodrug; self-assembly by brief sonication and microfluidization, perfluorocarbon, glycerin, pH 6.5, at 20,000 psi for 4 min.
†Hydrodynamic diameter (dynamic light scattering [DLS]): 220 nm; electrophoretic potential: −17 mV; polydispersity index: 0.19.
‡Hydrodynamic diameter (DLS): 280 nm; electrophoretic potential: −22 mV; polydispersity index: 0.25.
¶Hydrodynamic diameter (DLS): 230 nm; electrophoretic potential: −21 mV; polydispersity index: 0.06.
§Hydrodynamic diameter (DLS): 280 nm; electrophoretic potential: −10 mV; polydispersity index: 0.08.