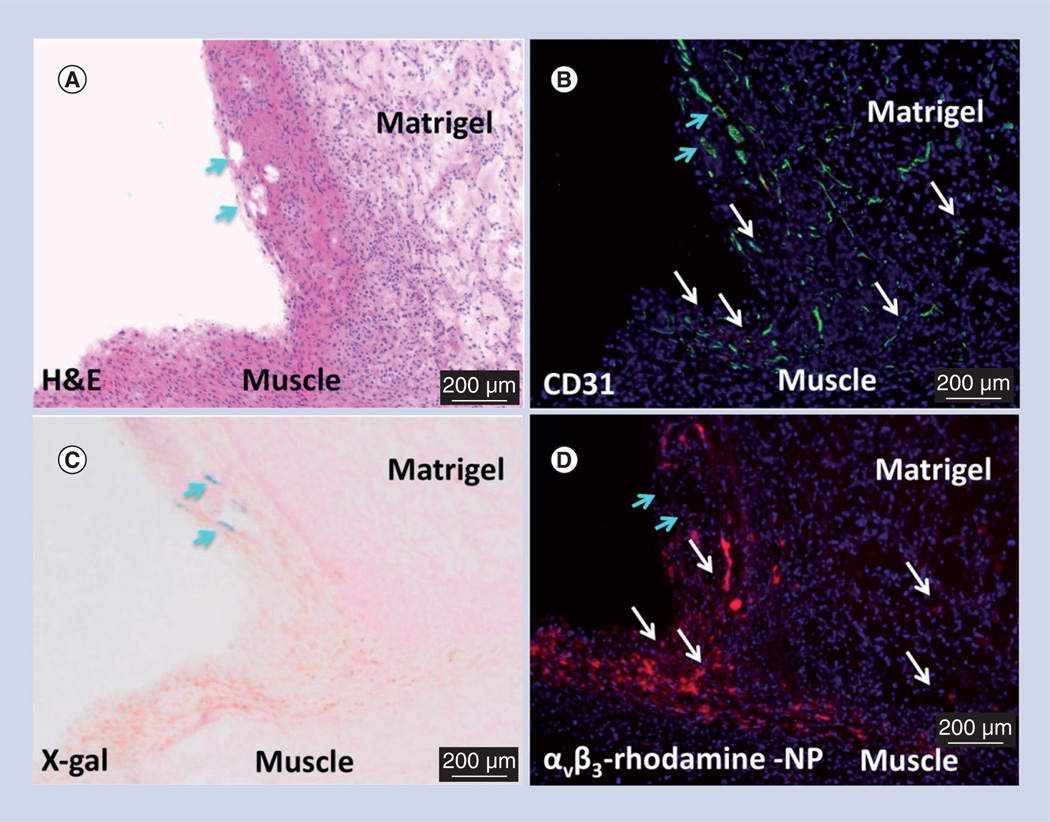
Figure 3. Microscopic examination of FGF Matrigel™ subcutaneous explant (serial sections) from FVB/N-TgN(TIE-2-LacZ)182-Sato mice following injection (intravenous) of αvβ3-targeted rhodamine-labeled perfluorocarbon nanoparticles.
(A) H&E staining to illustrate the anatomic location of the implant relative to the muscle, revealing a region with prominent mature vessels (arrows). Note proliferation of inflammatory cells along the muscle and implant interface. (B) PECAM (CD31) staining of endothelium revealing prominent vasculature along the Matrigel periphery and the development of microvessels within the implant. (C) LacZ signal for β-galactosidase under Tie-2 promoter control. Lac-Z staining (arrows) is appreciated in a region of more mature vessels along the implant periphery but the signal was not observed in most of the inflammatory regions along the muscle–implant interface or within the implant. (D) Binding of the αvβ3-targeted rhodamine-labeled perfluorocarbon nanoparticles densely within the inflammed tissue along the muscle and implant interface and diffusely within the Matrigel implant. Regions of αvβ3-targeted rhodamine-labeled perfluorocarbon nanoparticle binding is generally correlated with areas of PECAM staining (white arrows) except for an area of rhodamine signal dropout (blue arrows) in (D), which spatially paralleled prominent PECAM and LacZ staining in (B) and (C), respectively. These images corroborate the specific binding of αvβ3-targeted nanoparticles to nascent angiogenic vessels (Tie-2−, PECAM+) and not to maturing microvessels (Tie-2+, PECAM+).
H&E: Hematoxylin and eosin; NP: Nanoparticle.