Abstract
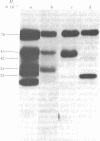
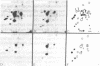
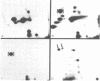
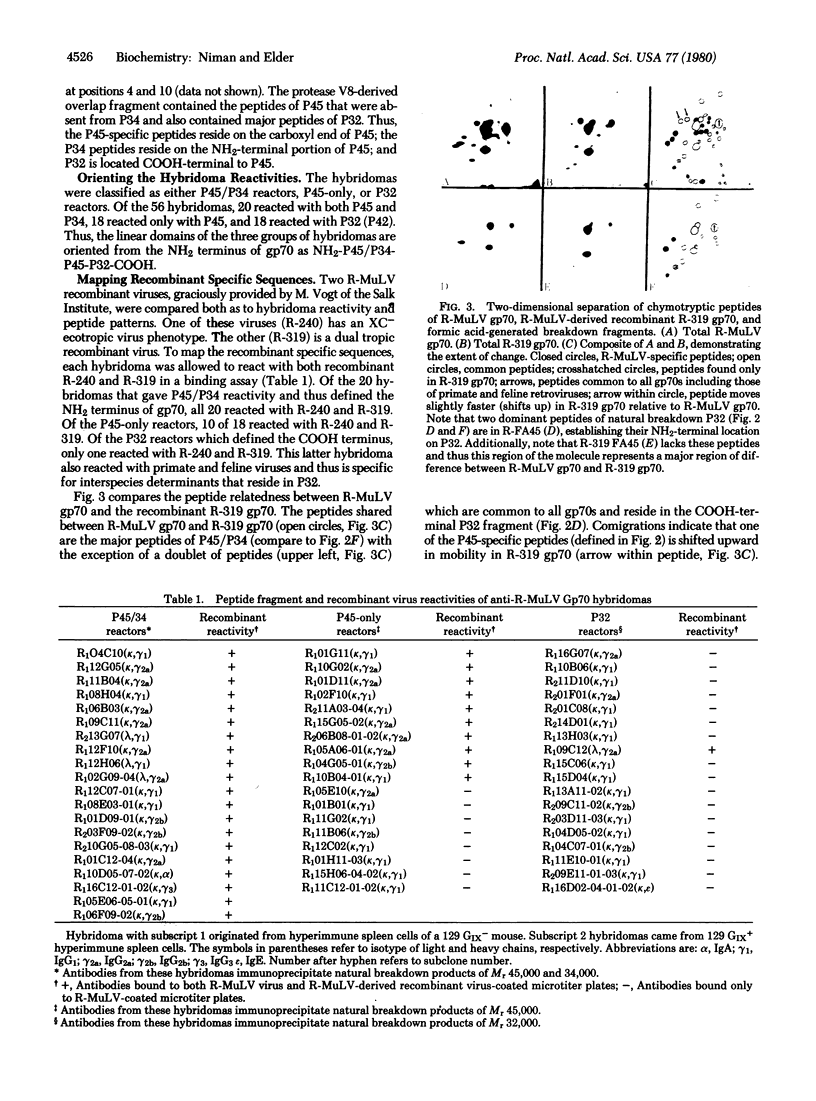
Using hybridoma-specific immune precipitations of fragments derived from Rauscher virus gp70, coupled with peptide patterns (fingerprinting) and partial amino acid sequence analyses, we have generated a linear map of Rauscher gp70. We used a panel of 56 hybridomas derived from the fusion of the drug-selected SP-2 myeloma line with spleen cells from either a 129 GIX+ or a GIX- mouse immunized with purified Rauscher virus gp70. The results showed that by "natural" breakdown, gp70 splits into predominant fragments, with Mr 45,000 (P45), 34,000 (P34), and 32,000 (P32). Peptide fingerprinting of these as well as overlapping fragments coupled with partial amino acid sequence analyses allowed us to align the fragments into the linear arrangement NH2-P45-P32-COOH, with P34 being an NH2-terminal degradation product of P45. Of the 56 hybridomas, 20 immunoprecipitated both P45 and P34; 18 immunoprecipitated only P45; and 18 immunoprecipitated only P32. The hybridomas thus define three domains of the molecule as NH2-P45/P34, P45 only, and P32-COOH. Allowing these hybridomas to react with two Rauscher-derived envelope gene recombinant viruses yielded the following results: (i) all 20 P45/34 reactors bound to the two Rauscher recombinants; (ii) of 18 P45-only hybridomas, 10 reacted; and (iii) only 1 of 18 P32 reactors bound to the Rauscher recombinants. This last hybridoma reacted with various murine retroviruses, indicating that it was directed at conserved determinants of gp70. Peptide fingerprinting of R-gp70, the recombinant gp70s, and their respective breakdown products confirmed the homologies and nonhomologies defined by the hybridomas. Furthermore, peptide patterns showed that these acquired sequences on the COOH-terminal portion of the recombinant gp70s are related to xenotropic virus gp70s.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosselman R. A., Van Griensven L. J., Vogt M., Verma I. M. Genome organization of retroviruses. VI. Heteroduplex analysis of ecotropic and xenotropic sequences of moloney mink cell focus-inducing viral RNA obtained from either a cloned isolate or a thymoma cell line. J Virol. 1979 Dec;32(3):968–978. doi: 10.1128/jvi.32.3.968-978.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. H., Verma I. M., Shih T. Y., Scolnick E. M., Davidson N. Heteroduplex analysis of the sequence relations between the RNAs of mink cell focus-inducing and murine leukemia viruses. J Virol. 1978 Oct;28(1):352–360. doi: 10.1128/jvi.28.1.352-360.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devare S. G., Rapp U. R., Todaro G. J., Stephenson J. R. Acquisition of oncogenicity by endogenous mouse type C viruses: effects of variations in env and gag genes. J Virol. 1978 Nov;28(2):457–465. doi: 10.1128/jvi.28.2.457-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Rothenberg E., Hopkins N., Baltimore D., Sharp P. A. Heteroduplex analysis of the nonhomology region between Moloney MuLV and the dual host range derivative HIX virus. Cell. 1978 Aug;14(4):959–970. doi: 10.1016/0092-8674(78)90350-1. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Jensen F. C., Bryant M. L., Lerner R. A. Polymorphism of the major envelope glycoprotein (gp70) of murine C-type viruses: virion associated and differentiation antigens encoded by a multi-gene family. Nature. 1977 May 5;267(5606):23–28. doi: 10.1038/267023a0. [DOI] [PubMed] [Google Scholar]
- Faller D. V., Rommelaere J., Hopkins N. Large T1 oligonucleotides of Moloney leukemia virus missing in an env gene recombinant, HIX, are present on an intracellular 21S Moloney viral RNA species. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2964–2968. doi: 10.1073/pnas.75.6.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Frankel A. E., Elder J. H., Lerner R. A., Ihle J. N., Bolognesi D. P. Biological, immunological, and biochemical evidence that HIX virus is a recombinant between Moloney leukemia virus and a murine xenotropic C type virus. Virology. 1978 Oct 15;90(2):241–254. doi: 10.1016/0042-6822(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Bolognesi D. P. A novel murine oncornavirus with dual eco- and xenotropic properties. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5150–5155. doi: 10.1073/pnas.72.12.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L., Margulies D. H., Scharff M. D. A simple method for polyethylene glycol-promoted hybridization of mouse myeloma cells. Somatic Cell Genet. 1977 Mar;3(2):231–236. doi: 10.1007/BF01551818. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Smythers G. W., Marquardt H., Oroszlan S. Amino-terminal amino acid sequence and carboxyl-terminal analysis of Rauscher murine leukemia virus glycoproteins. Virology. 1978 Mar;85(1):319–322. doi: 10.1016/0042-6822(78)90437-3. [DOI] [PubMed] [Google Scholar]
- Krantz M. J., Strand M., August J. T. Biochemical and immunological characterization of the major envelope glycoprotein gp69/71 and degradation fragments from Rauscher leukemia virus. J Virol. 1977 Jun;22(3):804–815. doi: 10.1128/jvi.22.3.804-815.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Gilden R. V., Oroszlan S. Envelope glycoproteins of Rauscher murine leukemia virus: isolation and chemical characterization. Biochemistry. 1977 Feb 22;16(4):710–717. doi: 10.1021/bi00623a024. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Pierce S. K., Klinman N. R. Antibody-specific immunoregulation. J Exp Med. 1977 Aug 1;146(2):509–519. doi: 10.1084/jem.146.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Troxler D. H., Coffin J. M., Scolnick E. M. Mapping host range-specific oligonucleotides within genomes of the ecotropic and mink cell focus-inducing strains of Moloney murine leukemia virus. J Virol. 1978 Apr;26(1):71–83. doi: 10.1128/jvi.26.1.71-83.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of oncogenic ribonucleic acid viruses. Interspec II, a new interspecies antigen. J Biol Chem. 1973 Aug 25;248(16):5627–5633. [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Verma I. M., Lerner R. A. Nucleotide sequence of Moloney leukemia virus: 3' end reveals details of replications, analogy to bacterial transposons, and an unexpected gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3302–3306. doi: 10.1073/pnas.77.6.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Conjugation of p-benzoquinone treated enzymes with antibodies and Fab fragments. Immunochemistry. 1977 Nov-Dec;14(11-12):767–774. doi: 10.1016/0019-2791(77)90352-4. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Lowy D., Howk R., Young H., Scolnick E. M. Friend strain of spleen focus-forming virus is a recombinant between ecotropic murine type C virus and the env gene region of xenotropic type C virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4671–4675. doi: 10.1073/pnas.74.10.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Yuan E., Linemeyer D., Ruscetti S., Scolnick E. M. Helper-independent mink cell focus-inducing strains of Friend murine type-C virus: potential relationship to the origin of replication-defective spleen focus-forming virus. J Exp Med. 1978 Sep 1;148(3):639–653. doi: 10.1084/jem.148.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M. Properties of "mink cell focus-inducing" (MCF) virus isolated from spontaneous lymphoma lines of BALB/c mice carrying Moloney leukemia virus as an endogenous virus. Virology. 1979 Feb;93(1):226–236. doi: 10.1016/0042-6822(79)90290-3. [DOI] [PubMed] [Google Scholar]
- van Griensven L. J., Vogt M. Rauscher "mink cell focus-inducing" (MCF) virus causes erythroleukemia in mice: its isolation and properties. Virology. 1980 Mar;101(2):376–388. doi: 10.1016/0042-6822(80)90451-1. [DOI] [PubMed] [Google Scholar]