Table 1.
Activity of Salicylanilides against T. gondii
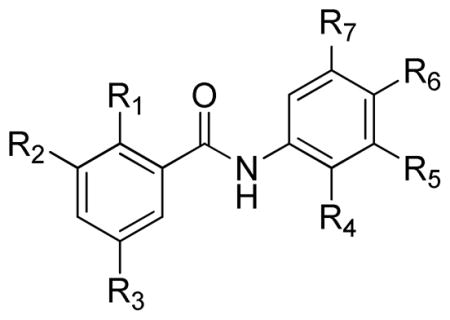
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | R5 | R6 | R7 | MIC50 | MIC90 |
| 3a | OH | H | Cl | H | CH3 | H | H | >1 μM | >1 μM |
| 3b | OH | H | Cl | H | Br | H | H | 750-500 nM | 1 μM–750 nM |
| 3c | OH | H | Cl | H | CH2CH3 | H | H | 500-250 nM | 750-500 nM |
| 3d | OH | H | Cl | H | C≡CH | H | H | >1 μM | >1 μM |
| 3e | OH | H | Cl | H | CH=CH2 | H | H | 500-250 nM | 1 μM–750 nM |
| 3f | OH | H | Cl | H | CF3 | H | H | 500-250 nM | 500-250 nM |
| 3g | OH | H | Cl | H | CN | H | H | >1 μM | >1 μM |
| 3h | OH | H | Cl | H | F | H | H | 570-500 nM | 1 μM–750 nM |
| 3i | OH | H | Cl | H | C(CH3)3 | H | H | 16-8 nM | 31-16 nM |
| 3j | OH | H | Cl | H | CF3 | H | CF3 | 31-16 nM | 250-125 nM |
| 3k | OH | H | H | Cl | H | NO2 | H | >1 μM | >1 μM |
| 3l | OH | H | Cl | H | CH2Ph | H | H | 1 μM–750 nM | 1 μM–750 nM |
| 3m | OH | H | CH3 | Cl | H | NO2 | H | 500-250 nM | 500-250 nM |
| 3n | OH | H | Cl | H | Cl | H | Cl | 500-250 nM | 500-250 nM |
| 3o | OH | H | Cl | H | F | H | F | >1 μM | >1 μM |
| 3p | OH | H | Cl | F | H | Cl | H | 750-500 nM | 750-500 nM |
| 3q | OH | H | Cl | OCH3 | OCH3 | OCH3 | H | >1 μM | >1 μM |
| 3r | OH | H | Cl | Cl | H | H | CN | 750-500 nM | 1 μM–750 nM |
| 3s | OH | H | Cl | H | CH3 | H | CH3 | >1 μM | >1 μM |
| 3t | OH | H | Cl | H | C≡CH | F | H | >1 μM | >1 μM |
| 3u | OH | H | Cl | H | OCH2CH3 | H | H | >1 μM | >1 μM |
| 3v | OH | H | Cl | H | OCH3 | OCH3 | H | >1 μM | >1 μM |
| 3w | OH | H | Cl | H | OCH(CH3)2 | H | H | >1 μM | >1 μM |
| 3x | OH | H | Cl | H | OCH3 | H | CH3 | >1 μM | >1 μM |
| 3y | OH | H | Cl | H | OPh | H | H | >1 μM | >1 μM |
| 3z | OH | H | Cl | H | OCH(CF3)2 | H | H | >1 μM | >1 μM |
| 3aa | OH | I | I | H | Cl | H | H | >1 μM | >1 μM |
| 3ab | OH | H | Cl | H | OCH2CH2O | H | >1 μM | >1 μM | |
| 3ac | OH | H | F | H | OCH2CH3 | H | H | >1 μM | >1 μM |
| 4 | OH | H | Cl | Cl | H | NO2 | H | 250-200 nM | 250-200 nM |
| 5 | OH | H | Cl | Cl | H | NH2 | H | >1 μM | >1 μM |
| 7a | PhC(=O)O | H | Cl | Cl | H | NO2 | H | 125-61 nM | 250-125 nM |
| 7b |

|
H | Cl | Cl | H | NO2 | H | >1 μM | >1 μM |
| 7c |
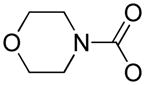
|
H | Cl | Cl | H | NO2 | H | >1 μM | >1 μM |
| 7d |

|
H | Cl | Cl | H | NO2 | H | 750-500 nM | 1 μM–750 nM |
| 10a | CH3O | H | F | H | CH3 | H | H | >1 μM | >1 μM |
| 10b | CH3O | H | F | H | OCH3 | H | H | >1 μM | >1 μM |
| 14a |

|
H | Cl | Cl | H | NO2 | H | 31-16 nM | 250-125 nM |
| 14b |

|
H | Cl | H | CF3 | H | CF3 | 250-125 nM | 250-125 nM |
