Abstract
The activity of the N2-fixing cyanobacterial genus Trichodesmium is critical to the global nitrogen (N) and carbon (C) cycles. Although iron (Fe) has been shown to be an important element limiting the growth and N2 fixation of Trichodesmium, there have been no specific data demonstrating the in situ affect of Fe on Trichodesmium. We surveyed Trichodesmium populations from the Atlantic and Pacific Oceans for Fe limitation using a novel quantitative reverse transcriptase-PCR (qRT-PCR) method monitoring the expression of an Fe limitation-induced gene, isiB. Here we report the first molecular evidence of in situ Fe limitation of Trichodesmium N2 fixation, which was evident in samples from the Pacific Ocean, whereas limitation appeared minimal to nonexistent in Atlantic Ocean samples. As our method is Trichodesmium clade specific, we were also able to determine that representatives from the Trichodesmium tenue clade were the most biologically active group of Trichodesmium in the majority of our samples, which speaks to their dominance in open ocean regimes. Furthermore, comparisons of our field expression and chemical data with laboratory studies suggest that the majority of dissolved Fe in the open ocean is available to Trichodesmium colonies regardless of Fe complexation.
Keywords: nitrogen fixation, diazotrophs, iron, marine, Cyanobacteria
Introduction
The N2-fixing cyanobacterial genus Trichodesmium is important to the global nitrogen (N) cycle by providing ‘new' N to the system through N2 fixation (Capone et al., 1997). Data synthesized from multiple studies in the Atlantic Ocean show that diazotrophy (N2 fixation) by Trichodesmium spp. can exceed mixing–driven NO3 flux into the euphotic zone (Capone et al., 2005), and it has been hypothesized that changes in N2 fixation from diazotrophs can potentially influence CO2 sequestration over geologic time scales (Capone et al., 1997; Falkowski, 1997; Gruber and Sarmiento, 1997). Additionally, recent work in multiple laboratories has shown that N2 fixation by Trichodesmium erythraeum IMS101 is predicted to increase concomitantly with atmospheric CO2 (Hutchins et al., 2007; Levitan et al., 2007; Ramos et al., 2007). Therefore, defining the factors that affect Trichodesmium activity in the modern ocean is important for forecasting the biological removal of CO2 from the atmosphere in the future.
The Trichodesmium genus comprises two major clades with cultured representatives (hereafter abbreviated T. erythraeum (Tery) and Trichodesmium tenue (Tten)), and although there are other Trichodesmium sequences that are episodically detected in field samples (Janson et al., 1999; Orcutt et al., 2002; Lundgren et al., 2005; Hynes, 2009), members of these two major clades are the most commonly described (Benporath et al., 1993; Carpenter et al., 1993; Dyhrman et al., 2006; Hynes, 2009; Hynes et al., 2009). Despite the importance of Trichodesmium, the factors driving speciation in the genus have yet to be defined. In the last decade, a number of key laboratory discoveries have been made regarding the growth and N2-fixation rates of Trichodesmium, including: the CO2 effects mentioned above, physical forcing from light (Breitbarth et al., 2008) as well as defining the temperature range of a representative of the Tery clade (Breitbarth et al., 2007). Additionally, other laboratory/field work (Berman-Frank et al., 2001, 2007; Webb et al., 2007; Jakuba et al., 2008; Chappell and Webb, 2010), chemical correlations in the field (Sanudo-Wilhelmy et al., 2001; Kustka et al., 2003b; Moore et al., 2009) and community incubations (Mills et al., 2004) show that the elements iron (Fe) and phosphorus (P) can influence Trichodesmium growth and N2-fixation capabilities. Because of its low solubility and comparatively high cellular requirement in Trichodesmium (Wu et al., 2001; Kustka et al., 2003b), Fe has been hypothesized to be an important limiting factor for N2 fixation in much of the oceans that Trichodesmium inhabits (Berman-Frank et al., 2001), potentially controlling large-scale distributions of N2 fixation (Moore et al., 2009). However, this predicted, widespread Fe limitation has yet to be directly demonstrated in situ, and it remains unclear what form of Fe is truly bioavailable for Trichodesmium.
Dissolved Fe (dFe) in sea water is operationally defined as the Fe that can flow through a 0.2 or 0.4 μm filter (Cutter et al., 2010), and it is well established that in the surface oceans >99% of this Fe fraction is bound to organic ligands with undefined structures and uncertain bioavailability (Rue and Bruland, 1995). The remaining ⩽1% of dFe (denoted Fe') is often presumed to be bioavailable and predominantly comprises Fe bound to inorganic ligands, and a small amount of the free ion (Fe3+) (Hudson and Morel, 1993). Potentially less bioavailable colloidal Fe (that is, Fe that is retained on a 0.02-μm filter) can also occur within the dFe fraction, but it has been suggested that most, if not all, noncolloidal forms are available to biology (Wu et al., 2001). However, as the influence of organic complexation on Fe availability to phytoplankton is still uncertain, it is generally ignored in biogeochemical models (Moore et al., 2004; Coles and Hood, 2007; Krishnamurthy et al., 2007; Moore and Doney, 2007).
There are multiple methods to measure Fe stress and growth limitation in oceanic phytoplankton (Kolber et al., 1994; Mills et al., 2004; Rivers et al., 2009). For example, the Fe stress-specific induction of flavodoxin has proven to be a valuable diagnostic in phytoplankton (LaRoche et al., 1996). Building on this work, we developed an N2-fixation-rate-calibrated, clade-specific (that is, Tten and Tery), quantitative reverse transcriptase-PCR (qRT-PCR) method to evaluate Fe limitation of N2 fixation in Trichodesmium spp. by monitoring the expression of the gene orthologs encoding flavodoxin, isiB (Chappell and Webb, 2010). We applied this assay on Trichodesmium colonies collected during daytime from three cruises in both the Atlantic and Pacific Oceans and analyzed the results in the context of in situ measurements of dFe and dissolved inorganic P (DIP). We chose sampling times and locations that were anticipated to cover a range of dFe and DIP concentrations, thus including regions where we expected to find Fe limitation as well as those where we hypothesized Trichodesmium would likely be P limited or Fe/P co-limited. Our data show that Fe is indeed limiting to Trichodesmium in the ocean. Additionally, comparisons of our field expression data with laboratory results suggest that most, if not all, of the dFe is bioavailable to Trichodesmium regardless of complexation.
Materials and methods
Sample collection
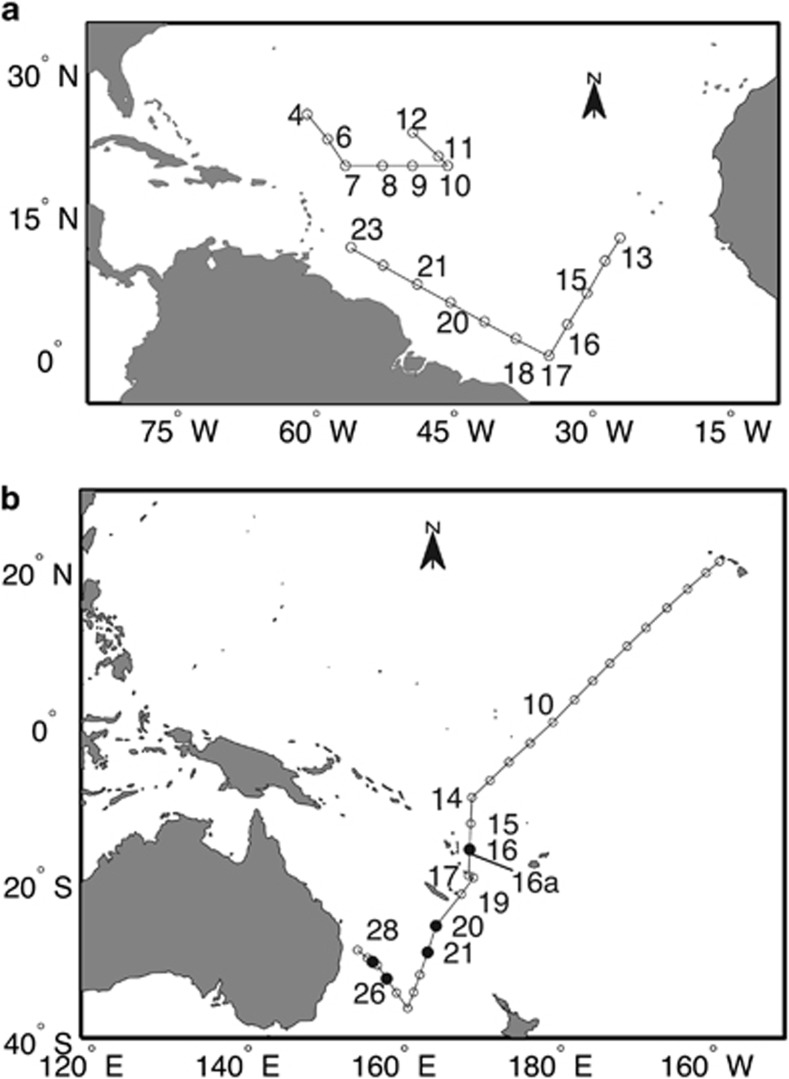
Sampling took place on three cruises: in the Sargasso Sea on the R/V Oceanus (cruise OC399-4), 22–30 March 2004 (Figure 1a, stations 4–12), in the Equatorial Atlantic aboard the R/V Seward Johnson (cruise SJ0609), 12–24 July 2006 (Figure 1a, stations 13–21), and aboard the R/V Kilo Moana (cruise KM0701) as part of the Western Pacific Warm Pool cruise, 12 January to 9 February 2007 (Figure 1b). Samples for dFe and nutrients were collected using standard trace metal techniques similar to those described by Jakuba et al. (2008) using acid-cleaned 10 l Teflon-coated Go-Flo bottles (General Oceanics, Miami, FL, USA) or 5 l Teflon-coated exterior spring Niskin bottles (Ocean Test Equipment, Ft. Lauderdale, FL, USA) deployed on a Kevlar line. All sample processing was done under Class 100 cleanroom conditions either in a trace metal clean ‘bubble' kept at positive pressure using HEPA (high-efficiency particulate arresting)-filtered air flow that was built on the ship or a trace metal clean van also supplied with HEPA-filtered air. Water for dFe analysis was 0.4-μm filtered, collected in acid-cleaned 250 ml low-density polyethylene bottles and acidified to pH 1.7 with concentrated high-purity hydrochloric acid (Seastar, Sidney, BC, Canada). The value of dFe in the seawater samples was determined using isotope dilution and magnesium hydroxide pre-concentration followed by analysis using inductively coupled mass spectrometry (Saito and Schneider, 2006).
Figure 1.
Map of cruise tracks. (a) OC399-4 (stations 4–12) in the Sargasso Sea in March 2004. SJ0609 (Stations 13–21) east-to-west transect across the equatorial Atlantic Ocean in July 2006. (b) KM0701 north-to-south transect through the Western Pacific Warm Pool during January and February 2007. Open circles mark all stations from each cruise. Numbered stations represent where Trichodesmium colonies were collected. Filled circles represent where isiB expression was above threshold value indicative of Fe limitation.
Water for nutrient analysis was 0.4-μm filtered and then immediately frozen at −20 °C for later analysis. Analysis of DIP concentrations from the Sargasso Sea cruise was reported by Jakuba et al. (2008). Samples from SJ0609 and KM0701 were sent to the College of Oceanic and Atmospheric Sciences at Oregon State University where DIP was analyzed using a Technicon AutoAnalyzer II (SEAL Analytical Ltd, Fareham, UK) by J Jennings with a detection level of 6 nmol l−1.
Trichodesmium samples were collected using a 130-μm phytoplankton net (Sea-Gear Corporation, Melbourne, FL, USA) that was hand towed at the surface for 10–20 min using a 30-m line. Immediately following the return to the surface, the contents of the tow were taken into the air-conditioned laboratory aboard the ship where the Trichodesmium colonies were separated from the other plankton using polypropylene bulb transfer pipettes. Colonies were transferred from the bulk solution into clean 0.4 μm-filtered microwave-sterilized sea water, then they were filtered onto 5-μm polycarbonate filters and stored in liquid N2 until RNA processing and analysis. Because of the relatively high Trichodesmium biomass observed on OC399-4, 200 ml of the bulk net tow was filtered onto 5-μm polycarbonate filters and preserved in liquid N2 without separation and rinsing. On cruises OC399-4 and KM0701, samples were always taken between 0800 and 1000 h local time, which is consistent with the timing of sampling in our laboratory study (Chappell and Webb, 2010); on cruise SJ0609, samples were taken throughout the day and night (Supplementary Table S1), but only samples obtained during the day were included in later expression analyses.
RNA extraction and qRT-PCR
Upon return to the laboratory, samples were processed and analyzed using the previously described procedure for RNA extraction and qRT-PCR analysis of gene expression (Chappell and Webb, 2010). Separate primer sets for the Tery clade targeting T. erythraeum strains (IMS101 and GBRTRLI101) and T. contortum strain (21–74) and the Tten clade targeting T. tenue strains (Z-1 and H9-4) and T. theibautii strain (VI-I) were designed for orthologs of both our target Fe stress response gene, isiB, and a RNA normalization control gene, rnpB. The primers were originally described by Chappell and Webb (2010) and are relisted in Supplementary Table S2.
Cloning
Prior laboratory work had shown that the clade-specific isiB and rnpB primers only amplified products from the expected isolates (Chappell and Webb, 2010), but to verify this finding with field samples, we cloned Tten RT-PCR products from two stations during the KM0701 cruise: Stations WP14 and WP16a. The PCR for cloning was done using an ABI9700 PCR System (Applied Biosystems Inc., Foster City, CA, USA), which has the same ramp settings as the 7500 Fast Real-Time PCR Machine using MasterTaq Taq DNA polymerase (Eppendorf AG, Hamburg, Germany), at a final concentration of 1.25 units per PCR reaction as per the manufacturer's instructions without additional Mg+2 (1 × ) in a 20 μl reaction with a final complementary DNA (cDNA) concentration of 1–2 nmol l−1 and a final primer concentration of 200 nmol l−1. To mimic the qRT-PCR conditions, the PCR cycling conditions were 50 °C for 2 min, 95 °C for 10 min; 40 cycles of 95 °C for 15 s and 55 °C for 1 min; followed by a final extension at 55 °C for 5 min. The RT-PCR products from Stations WP16a and WP14 amplified with Tten isiB and Tten rnpB primers were cloned directly using the TOPO TA Cloning Kit for Sequencing (Invitrogen Corporation, Carlsbad, CA, USA). A total of 48 clones from each reaction were incubated in 2 × LB for 36 h at 28 °C in a shaking incubator (New Brunswick Scientific, Edison, NJ, USA) and extracted using the Montage Plasmid Miniprep96 Kit (Millipore Corporation, Billerica, MA, USA) as per the manufacturer's guidelines and sequenced using M13 forward and reverse primers by Laragen Inc. (Los Angeles, CA, USA). Sequences were trimmed of vector and examined. As all rnpB sequences were identical to T. tenue rnpB, no further analysis was done. The isiB sequences were aligned with Trichodesmium isiB sequences from GenBank using the MUSCLE algorithm (Edgar, 2004) and then arranged into a phylogenetic tree using the maximum likelihood method (HKY85 substitution model, 1000 bootstraps, and transition/transversion of four) with PHYML in the Geneious software package (Biomatters, Auckland, New Zealand). As we rarely detected Tery in the field samples and there is reduced sequence diversity in this clade (Hynes et al., 2012), the Tery primers were not similarly tested.
Restriction analysis
Using the Tten isiB sequences defined above from stations WP16 and WP14, we were able to screen our RT-PCR products from all stations for the presence of uncultured, novel Trichodesmium Tten phylotypes in our amplicons from other stations via the presence of two separate, conserved restriction sites (Supplementary Figure S1). Briefly, cDNA was amplified from all KM0701 and SJ0609 stations (where prior qRT-PCR detected Tten isiB) using Tten isiB primers at a concentration of 200 nmol l−1, BIO-X-ACT Short Mix (Bioline, London, UK) at 1 × concentration in a 20 μl reaction with 1–2 nmol l−1 template on a Mastercycler thermal cycler (Eppendorf AG). The PCR cyling conditions were: 50 °C for 2 min, 95 °C for 10 min; 40 cycles of 95 °C for 15 s, 55 °C for 1 min; followed by a final extension at 60 °C for 5 min. For the HaeIII restriction digest, 5 μl of PCR product was incubated with 25 units of HaeIII (New England Biolabs, Ipswich, MA, USA) and 1 × NEB4 buffer in a final volume of 47.5 μl for 3 h at 37 °C, followed by 20 min at 80 °C. For the AclI restriction digest, 5 μl of PCR product was incubated with 7.5 units of AclI (New England Biolabs) and 1 × NEB4 buffer in a final volume of 45 μl for 3 h at 37 °C. Uncut and cut PCR products were run side by side on a 1.5% agarose gel and imaged using GeneSnap imaging software on a ChemiGenius2 gel documentation system (Synoptics Ltd, Cambridge, UK).
Nitrogen fixation analysis
The N2-fixation rates were measured on samples from two stations on cruise OC399-4 and six stations on KM0701 by the acetylene reduction assay described by Capone (1993) using the same methods described by Webb et al. (2007). The N2-fixation rates were normalized to flourometrically measured chlorophyll a (Herbland et al., 1985). Briefly, the protocol entailed incubating 10–20 colonies at temperatures similar to the sea water they were isolated from at ∼75 μmol quanta m−2 s−1 in 75 ml polycarbonate bottles (Nalge Nunc International Corp., Rochester, NY, USA) filled with 30 ml presterilized filtered sea water in a Percival incubator (Percival Scientific, Inc., Perry, IA, USA). A total of 6 ml of acetylene gas was added to the headspace at the start of the incubation, and acetylene production was measured shipboard on a Shimadzu GC-8a (Shimadzu Scientific Instruments, Columbia, MD, USA) hourly for a maximum of 3 h.
Results
Sampling and environmental parameters
A map of the sampling locations for all cruises is shown in Figure 1. Only stations where there was amplifiable Trichodesmium cDNA are numbered. Fitting with the winter season in the northern hemisphere, Trichodesmium was primarily detected in net tows during the southern portions of the WP cruise. Detailed information on station locations, sea surface temperature, dFe, DIP, % Tten clade rnpB and Tten clade expression values are listed in Table 1. The dFe values were highest in the Sargasso Sea and lowest in the western Pacific Ocean, which is consistent with global atmospheric dust deposition data (Mahowald et al., 2009). Conversely, DIP values were lowest in the Sargasso Sea and highest in the western Pacific Ocean. The Tten clade rnpB dominated most samples, and Tten isiB expression was lowest in the Sargasso Sea and highest in the western Pacific Ocean (Table 1).
Table 1. Near-surface (10–15 m) data from stations on all three cruises where isiB expression of Trichodesmium was measured.
| Station | Lat | Long | Temp (°C) | Fe (nM) | PO4 (μM) | Log (isiB/rnpB)a | %Tten |
|---|---|---|---|---|---|---|---|
| S4 | 25.40 | −61.15 | 24.7 | 1.17±0.04 | 0.0037±0.0049b | −1.96±0.02 | 99.95 |
| S6 | 22.80 | −58.93 | 25.2 | 1.04±0.08 | <0.014b | −2.30±0.02 | 99.97 |
| S7 | 20.00 | −57.00 | 25.0 | 1.15±0.09 | 0.0136±0.0049b | −2.34±0.04 | 99.99 |
| S8 | 20.00 | −52.97 | 25.2 | 1.00±0.12 | 0.0017±0.0001b | BDL | 100.00 |
| S9 | 20.00 | −49.73 | 25.0 | 1.11±0.04 | 0.002±0.0004b | −3.42±0.09 | 100.00 |
| S10 | 20.00 | −45.90 | 24.6 | 1.01±0.04 | 0.0058±0.0049b | BDL | 100.00 |
| S11 | 20.97 | −46.90 | 24.8 | 0.94±0.06 | 0.0016±0.0004b | −2.21±0.02 | 99.76 |
| S12 | 23.52 | −49.68 | 25.4 | 0.82±0.05 | 0.0033±0.0004b | −2.58±0.09 | 100.00 |
| E13 | 12.40 | −27.20 | 26.9 | 0.74±0.12 | 0.045±0.000 | −1.62±0.02 | 100.00 |
| E15 | 6.60 | −30.80 | 28.4 | 0.61±0.02 | 0.016±0.002 | −1.99±0.02 | 100.00 |
| E16 | 3.30 | −32.90 | 27.8 | 0.09±0.00 | 0.037±0.002 | −1.40±0.04 | 100.00 |
| E17 | 0.01 | −34.90 | 26.8 | 0.14±0.01 | 0.076±0.004 | −1.31±0.02 | 100.00 |
| E18 | 1.80 | −38.50 | 28.2 | 0.10±0.00 | 0.031±0.009 | −1.01±0.02c | 100.00 |
| E20 | 5.60 | −45.60 | 28.5 | 0.67±0.03 | 0.035±0.010 | −1.80±0.04 | 99.48 |
| E21 | 7.50 | −49.20 | 28.7 | 1.89±0.03 | 0.068±0.009 | −1.54±0.04 | 99.86 |
| E23 | 11.40 | −56.40 | 28.4 | 1.17±0.05 | 0.052±0.00 | −1.24±0.02c | NA |
| WP10 | 0.37 | −179.64 | 29.3 | 0.11±0.04 | 0.324±0.000d | 0.33±0.05a,c | 41.19 |
| WP14 | −9.25 | 170.00 | 30.6 | 0.20±0.04 | 0.168±0.000d | −1.98±0.02 | 100.00 |
| WP15 | −12.58 | 169.86 | 30.2 | 0.11±0.03 | 0.133±0.002d | −1.72±0.01 | 99.95 |
| WP16 | −15.89 | 169.72 | 28.8 | 0.29±0.03 | 0.169 ±0.000d | −0.14±0.04a | 99.61 |
| WP16a | −15.98 | 169.77 | 28.5 | 0.63±0.02 | 0.148±0.003d | −0.70±0.05a,c | 83.06 |
| WP17 | −19.23 | 169.58 | 27.2 | 0.95±0.02 | 0.137±0.004d | −1.66±0.03 | 99.86 |
| WP19 | −21.62 | 168.66 | 26.8 | 0.50±0.08 | 0.073±0.000d | −1.29±0.02 | 98.87 |
| WP20 | −25.67 | 165.42 | 26.0 | 0.09±0.02 | 0.102±0.002d | −0.56±0.05i | 99.59 |
| WP21 | −29.04 | 164.34 | 24.5 | 0.24±0.02 | 0.050±0.002d | 0.13±0.04a | 100.00 |
| WP26 | −32.42 | 159.09 | 24.5 | 0.20±0.02 | 0.084±0.002d | −0.63±0.03a | 99.98 |
| WP28 | −30.26 | 157.30 | 24.7 | 0.51±0.12 | 0.061±0.000d | −0.75±0.10a | NA |
Abbreviations: BDL, isiB expression was below detection level in the sample; Fe, iron; NA, not applicable; Tten, Trichodesmium tenue. ‘S' stations are from cruise OC399-4 in the Sargasso Sea, ‘E' stations are from cruise SJ0609 in the Equatorial region of the Atlantic Ocean and ‘WP' station are from cruise KM0701 in the Pacific Ocean.
The s.d. of triplicate technical replicates are given for [Fe], the isiB/rnpB ratio, and [PO4] from OC399-4 (S) and duplicate technical replicates for [PO4] from SJ0609 (E) and KM0701 (WP).
%Tten = ((no. of copies of Tten clade rnpB)/(no. of copies of Tery + Tten clade rnpB)) × 100.
Values above −0.84±0.16 are indicative of Fe limitation.
PO4 values from Jakuba et al. (2008).
Stations that were excluded from subsequent analysis because of primer specificity or time of sampling.
PO4 values from Hynes et al. (2009).
Q-PCR primer efficiency and specificity
Clade-specific primers were tested with multiple representatives of each clade (T. erythraeum strains IMS101 and GBRTRLI101 for the Tery primers and T. tenue strains Z-1 and H9-4 and T. thiebautii VI-I for the Tten primers). The efficiencies of the Tten isiB and Tten rnpB primers were between 90% and 94% using DNA isolated from cultures of T. tenue and T. thiebautii. The qPCR efficiencies for all qRT-PCRs run were generally in the 91–93% range, with three at 96% and one at 90%.
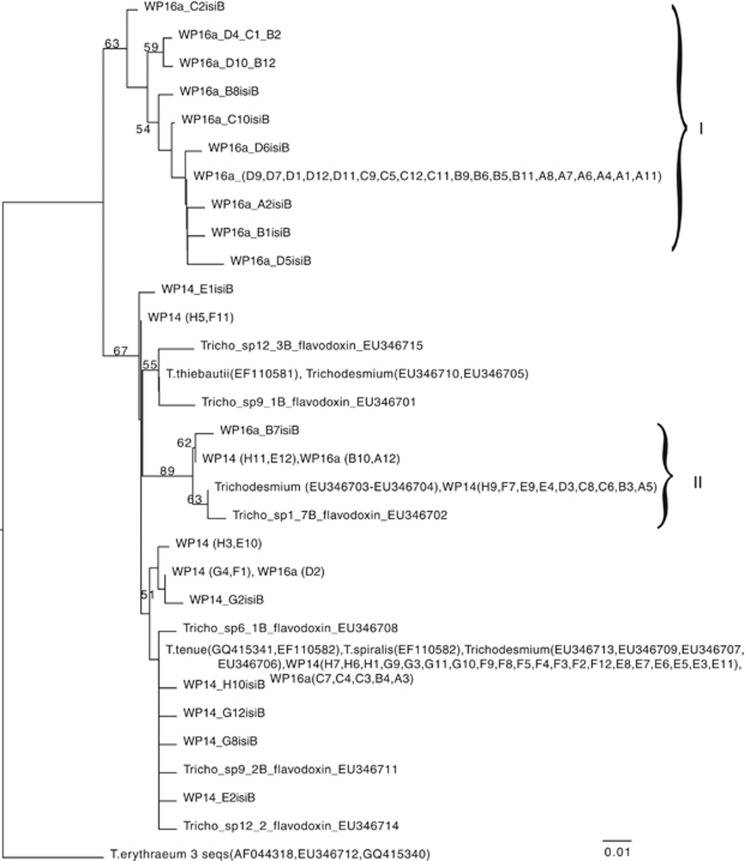
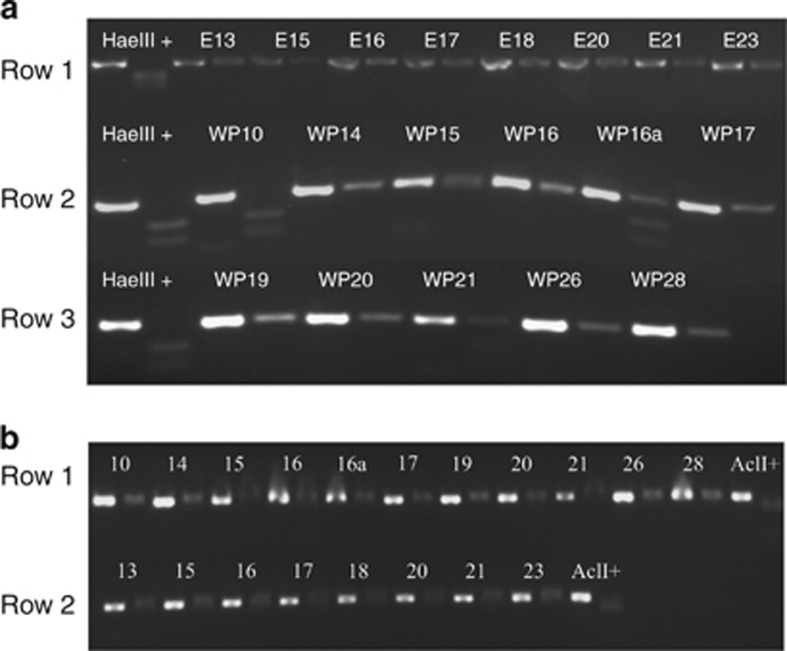
Although we have previously shown that the Trichodesmium rnpB and isiB Q-PCR primers were clade specific with DNA and RNA from cultured isolates (Chappell and Webb, 2010), Trichodesmium sequences have been detected in environmental studies that are not represented in culture (Janson et al., 1999; Lundgren et al., 2005; Hynes, 2009). To eliminate concerns that these potential uncultured phylotypes were more numerically dominant and/or might have different physiologies than our cultured isolates, we tested the specificity of our primers to verify that they were only amplifying the expected targets. Cloning and sequencing of the Tten clade rnpB Q-PCR products amplified from field samples yielded only sequences identical to T. tenue H9-4 (EMBL accession HE608976-HE609006), whereas this analysis of Tten clade isiB (EMBL accession HE608683-HE608765) revealed sequence diversity and showed that the primers were amplifying uncharacterized, noncultured phylotypes of Trichodesmium. Figure 2 and Supplementary Figure S2 summarize this analysis and identify a well-supported isiB clade from Station WP16a that is not represented in our culture collection and has not been seen before in field samples (group I on the tree), as well as a sub-clade with slightly lower boot-strap support values (group II on the tree). Examination of the alignment revealed a conserved HaeIII cut site 66 base pairs into the novel isiB PCR product of the group I sequences, and a conserved AclI cut site 40 base pairs into the novel isiB PCR product of the group II sequences that were not present in the rest of the Tten sequences (Supplementary Figures S1 and S2). Restriction analyses verified that sequences matching the new clade from Station WP16a (group I in Figure 2) were only detected in samples from stations WP16a and WP10 (Figure 3a), whereas the novel clade, group II in Figure 2, was not significantly represented in the PCR products from any station (Figure 3b). As cultured representatives of these newly identified Trichodesmium isiB clades do not exist, we were not able to test their Fe stress response, and thus we excluded isiB/rnpB expression data of stations WP10 and WP16a from subsequent analyses.
Figure 2.
Maximum likelihood tree of sequences from clones of Tten isiB RT-PCR products from two stations from the Pacific Ocean and Trichodesmium isiB sequences in Genbank based on a MUSCLE (Edgar, 2004) alignment done in Geneious (www.geneious.com) using the JC69 method of nucleotide substitution. Bootstrap values <50 were removed from the tree for clarity. Sequences in the ‘I' grouping have the HaeIII cut site. Sequences in the ‘II' grouping have the AclI cut site.
Figure 3.
(a) Image of a 1.5% Agarose gel of isiB RT-PCR products from field samples and HaeIII restriction digests. The left-most pair of each row is the isiB PCR product of a HaeIII-positive isiB clone and the result of the HaeIII restriction digest of that PCR product. Row 1 shows the results from the cDNA from the equatorial Atlantic transect (SJ0609) and rows 2 and 3 are the results from the cDNA from the western Pacific transect (KM0701). (b) Image of a 1.5% Agarose gel of isiB RT-PCR products from field samples and AclI restriction digests. The right-most pair of each row is the isiB PCR product of an AclI-positive isiB clone and the result of the AclI restriction digest of that PCR product. Row 1 shows the results from the cDNA from the western Pacific transect (KM0701) and row 2 shows the results from the cDNA from the equatorial Atlantic transect (SJ0609).
Fe stress in natural Trichodesmium samples
As mentioned above, there are many species of Trichodesmium in the ocean making up at least two clades (Janson et al., 1999; Lundgren et al., 2005; Hynes et al., 2012). Herein, we only report Tten clade-specific isiB gene expression (relative to the Tten reference gene rnpB), as the majority of samples did not have a significant amount of Tery clade RNA (Table 1).
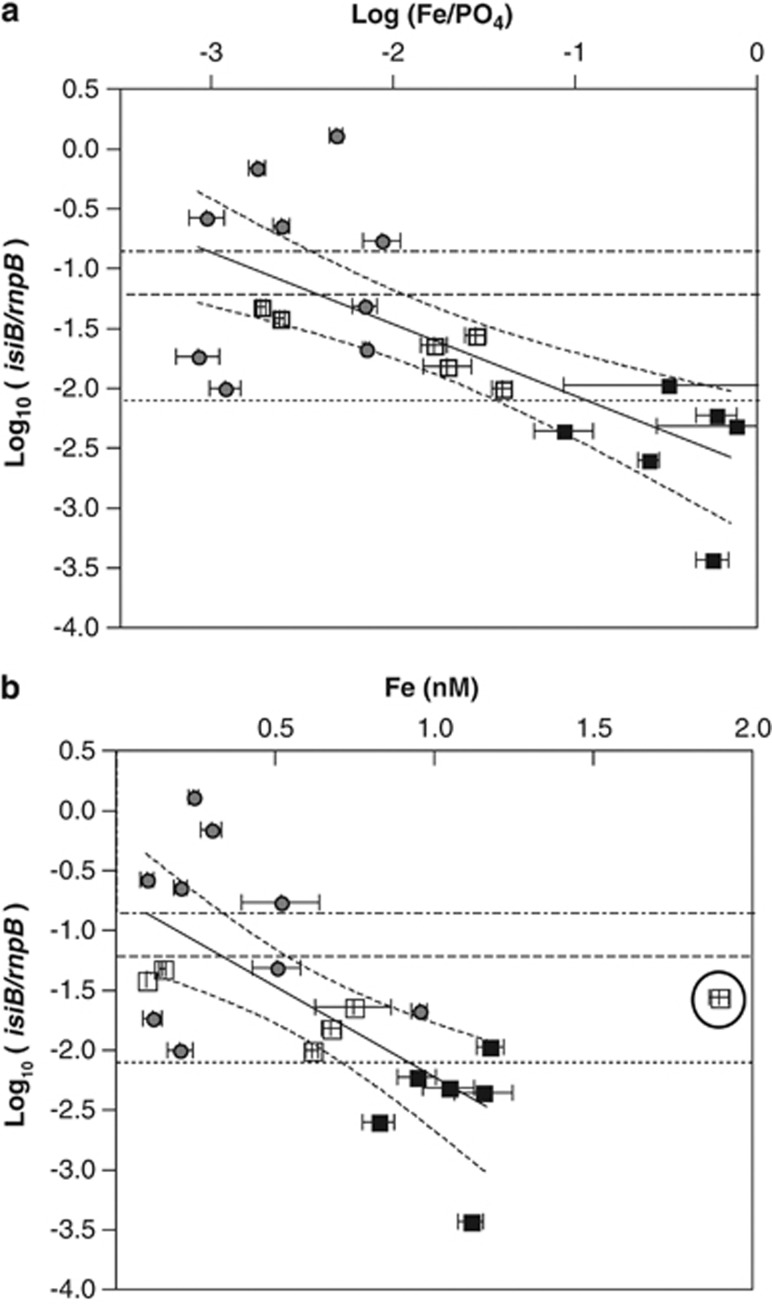
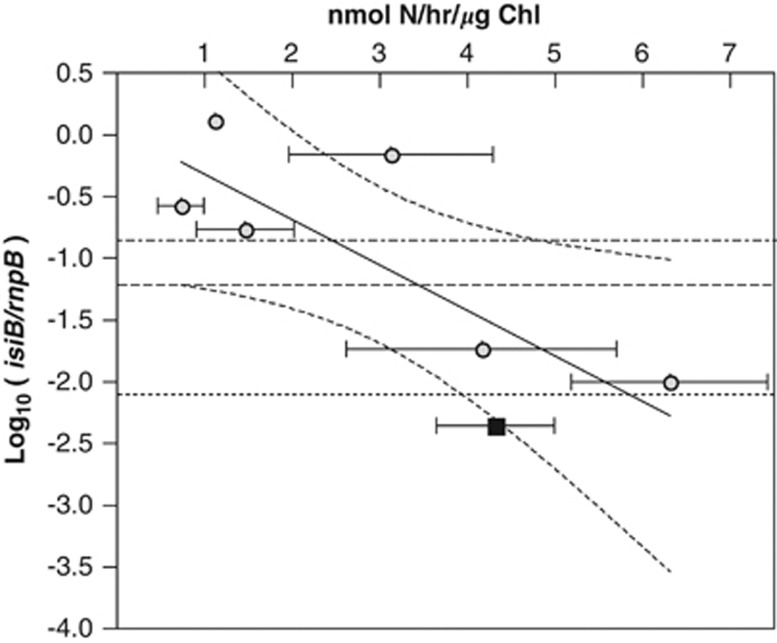
High expression of the Fe stress gene, isiB, was only detected in Tten colonies isolated from the Pacific and not from the Atlantic Ocean (Table 1). Comparisons of the Tten isiB expression data and dFe/DIP are depicted in Figure 4a and resulted in a line (equation: log10 (isiB/rnpB)=(−0.60±0.14) × log10 ([Fe]/[PO4])+(−2.7±0.28)) with an R2 value of 0.50. Comparisons of log10 (isiB/rnpB) and total dFe resulted in a line (equation: log10 (isiB/rnpB)=(−1.51±0.38) × [Fe]+(−0.7±0.26)), which has an R2 value of 0.47. The relationship between isiB expression and N2-fixation rates from select stations in the Atlantic and Pacific Oceans is shown in Figure 5, and is described by a linear regression line (nmol N fixed per h per μg chl=(−0.37±0.13) × (log10 (isiB/rnpB))+(0.05±0.46)), which has an R2 value of 0.62.
Figure 4.
Relationships between log10 (isiB/rnpB) of the Tten clade and log10 ([Fe]/[PO4]) (a), and dissolved [Fe] (b). Three dashed horizontal lines are shown on each plot. The bottom line is at the value log10(isiB/rnpB)=−2.09, which is the value associated with basal expression in the Tten clade from previous work (Chappell and Webb, 2010). The middle line at the value log10(isiB/rnpB) =−1.20, which is the value associated with a 50% reduction in N2 fixation in the Tten clade from previous work that we use as a threshold value to indicate Fe limitation of N2 fixation in Tten (Chappell and Webb, 2010). The top line is at the value log10(isiB/rnpB)=−0.84, which is the highest expression value measured in Tten laboratory cultures (Chappell and Webb, 2010). Values from the Sargasso Sea are plotted as filled squares, values from the Equatorial Atlantic are plotted as open squares and values from the Pacific are plotted as open circles. The solid line in (a) represents the linear regression of log10 ([Fe]/[PO4]) verses log10 (isiB/rnpB) with the dashed curves on either side representing the 95% confidence intervals. The equation for this line is log10 (isiB/rnpB)=(−0.60±0.14) × log10 ([Fe]/[PO4]) + (−2.7±0.28), which has an R2 value of 0.50. The solid line in (b) represents the linear regression of [Fe] versus log10 (isiB/rnpB) excluding the data point that is circled, which was influenced by the Amazon River Plume (Station E21). The dashed curves on either side represent the 95% confidence intervals for this regression. The equation for this line is log10 (isiB/rnpB)=(−1.51±0.38) × [Fe]+(−0.7±0.26), which has an R2 value of 0.47. Error bars represent the s.d. of triplicate analyses.
Figure 5.
Relationships between log10 (isiB/rnpB) and N2-fixation rates at select stations. Three dashed horizontal lines are shown on the plot. The bottom line is at the value log10(isiB/rnpB)=−2.09, which is the value associated with basal expression in the Tten clade from previous work (Chappell and Webb, 2010). The middle line at the value log10(isiB/rnpB)=−1.20, which is the value associated with a 50% reduction in N2 fixation in the Tten clade from previous work that we use as a threshold value to indicate Fe limitation of N2 fixation in Tten (Chappell and Webb, 2010). The top line is at the value log10(isiB/rnpB)=−0.84, which is the highest expression value measured in the Tten clade from previous work (Chappell and Webb, 2010). Values from the Sargasso Sea are plotted as filled squares and values from the Pacific are plotted as open circles. The solid line represents the linear regression of N2-fixation rates versus log10 (isiB/rnpB) with the dashed curves on either side representing the 95% confidence intervals. The equation for this line is: nmol N fixed per h per μg chl=(−0.37±0.13) × (log10 (isiB/rnpB))+(0.05±0.46), which has an R2 value of 0.62.
Discussion
A number of studies have established the importance of Fe limitation to Trichodesmium N2 fixation in the laboratory (Berman-Frank et al., 2001; Webb et al., 2001; Fu and Bell, 2003; Kustka et al., 2003a, 2003b; Berman-Frank et al., 2007; Shi et al., 2007; Kupper et al., 2008; Chappell and Webb, 2010). Recently, these efforts have focused on developing molecular methods that can be used to evaluate Trichodesmium Fe limitation at the cellular level (Webb et al., 2001; Shi et al., 2007; Chappell and Webb, 2010). Global biogeochemical models can be useful tools to predict the past, current, and future activity of key functional groups in the ocean (Moore et al., 2004; Coles and Hood, 2007; Krishnamurthy et al., 2007; Moore and Doney, 2007); however, empirical verification is required to prove where Fe is constraining Trichodesmium N2 fixation and to determine what form of Fe (that is, total, free, or inorganically bound Fe) is bioavailable. The development of a calibrated molecular method to evaluate Fe limitation of N2 fixation in Trichodesmium spp. (Chappell and Webb, 2010) provided a technique capable of achieving this goal. This study represents the first quantitative assessment of Fe limitation in field populations of Trichodesmium through areas predicted to be Fe limited, P limited, and/or Fe–P co-limited.
Quantitative analyses of cDNA revealed that Tten clade representatives were the predominant species of Trichodesmium detected in colonies in the field (Table 1, %Tten column). These results are consistent with previous morphological studies that found that T. thiebautii, a representative from the Tten clade, is the dominant Trichodesmium in the open ocean (Carpenter and Price, 1977; Sohm et al., 2008). The qPCR analysis of DNA from bulk surface water samples from the CTD also supports our conclusion that the Tten clade dominated the surface waters of our sampling sites (Hynes, 2009). Only two stations had a significant amount of Tery cDNA: stations WP10 and WP16a. Station WP10 appeared to be influenced by equatorial upwelling based on high DIP values (Supplementary Table S3) and Station WP16a was a sample from a Trichodesmium bloom that was entrained in ‘normal' water chemistry conditions (that is, we were unable to chemically identify the cause of the bloom). In addition to having a significant amount of Tery clade cDNA, both of these stations also showed evidence of a third group of Trichodesmium, the sub-clade of Tten that was revealed by our cloning efforts (Figure 3a), indicating that both stations exhibited a high overall diversity of Trichodesmium species. As there was a significant amount of Tten cDNA at all stations, dominating all but one station, isiB expression refers specifically to Tten isiB expression throughout the discussion.
During the Atlantic cruises, there was no indication of Fe limitation of N2 fixation based on isiB expression (that is, expression was never above the threshold value log10 (isiB/rnpB)=−1.20±0.1 corresponding to a 50% reduction in N2 fixation; Chappell and Webb, 2010; Table 1 and Figure 4). Basal isiB expression in all samples from the Sargasso Sea was consistent with low DIP and high dFe (Table 1 and Supplementary Figure S3). Although isiB expression was not above the threshold expression value in any of the Atlantic Ocean samples, there was an increase in expression above the basal expression value in some samples from the Equatorial Atlantic cruise indicative of growing Fe stress (Figure 4). Overall, the data from the two Atlantic cruises are consistent with previous work suggesting that the diazotrophs in the North Atlantic Ocean are predominantly P stressed (Sanudo-Wilhelmy et al., 2001; Webb et al., 2007), but that parts of the equatorial North Atlantic have the potential to shift between P and Fe limitation (Mills et al., 2004).
In contrast to the North Atlantic, measured levels of isiB expression in Tten colonies from the Pacific Ocean indicated that Fe limitation was common over much of that transect where Trichodesmium was detected (Figure 1 and Table 1). These data are consistent with model predictions suggesting that Fe limits N2 fixation in this region (Moore et al., 2004; Krishnamurthy et al., 2007; Moore and Doney, 2007). There was some evidence of P stress detected during cruise KM0701 toward the western side of the Pacific Basin (Hynes et al., 2009), which is consistent with a previous study indicating that parts of the southwestern Pacific Ocean can be transiently P stressed (Moutin et al., 2005). Although the qualitative nature of the P-stress assay employed by Hynes et al. (2009) limits our ability to directly address Fe–P co-limitation during KM0701, our quantitative isiB expression data from Trichodesmium demonstrate for the first time the importance of Fe in these sectors of the Pacific Ocean.
Comparison of environmental chemical concentrations and Trichodesmium isiB expression data revealed an inverse linear relationship between isiB expression and dFe/DIP (R2=0.50; Figure 4a). Given the importance of both Fe and P as potential limiting nutrients for Trichodesmium (Berman-Frank et al., 2001; Sanudo-Wilhelmy et al., 2001; Kustka et al., 2003b), it is reasonable that there could be an association between isiB expression values and the dFe/DIP ratio. In a low dFe and DIP regime, Trichodesmium may be growing below its optimal rate because of P limitation, and thus may be able to satisfy its Fe requirement to support that slow growth without any induction of isiB (that is, no Fe limitation). If DIP increases but dFe remains low and constant, growth rates and Fe demand would increase, leading to Fe limitation and isiB expression. We are mindful that Trichodesmium can also access P from the dissolved organic P pool under conditions of P stress (Dyhrman et al., 2006; Sohm et al., 2008; Hynes et al., 2009). As the enzymes associated with dissolved organic P utilization are only significantly expressed when cells are DIP limited, we do not believe that dissolved organic P utilization would affect the relationship between isiB expression and dFe/DIP. In addition, there was no significant correlation between isiB expression and DIP (R2=0.25, data not shown). Linear regression analyses suggest that the dFe/DIP value associated with Fe limitation of N2 fixation in field populations of Trichodesmium is 0.004 mol mol–1, with a range from 0 to 0.011 mol mol–1 (propagation of error discussed in Supplementary Information). This range of values is similar to the dFe/DIP value of 0.008 mol mol–1, which can be derived from the ratio of the half-saturation constants for Fe and DIP uptake by Trichodesmium used to estimate the transition between Fe and P limitation in diazotrophs in the model of Krishnamurthy et al. (2007). Also, consistent with the model, we did not observe isiB expression above our threshold value indicative of Fe limitation at any station where the dFe/DIP ratio was >0.008 mol mol–1. Thus, our data can be interpreted as an empirical validation of the model parameters and predictions.
There was also an inverse linear relationship between Tten isiB expression and dFe (R2 value of 0.47) when Station E21, a station influenced by the Amazon River plume, was excluded from the analysis (Figure 4b). The influence of the Amazon River on Station E21 was evident in the low surface salinity (32.7 p.s.s.) and high dFe (2 nmol l−1). The elevated isiB expression at this station was surprising given this high dFe value. It is likely that the dFe at this station was bound by organic ligands that include a significant terrestrial component, as has been reported in other river plumes with similar salinities (Batchelli et al., 2010). The dFe complexes with such ligands may differ substantially in their bioavailability to Trichodesmium. Indeed, recent evidence suggests that fulvic acid isolated from the Suwanee River forms Fe complexes that are not bioavailable to another cyanobacterium, Synechococcus (Hassler and Twiss, 2006). Future work needs to examine isiB expression over a range of dFe concentrations in surface waters influenced by rivers. Using the equation derived from the linear regression of isiB expression and dFe, the critical dFe value associated with Fe limitation in the open ocean is 0.33±0.22 nmol l−1. This concentration is equivalent to the [Fe'] associated with Trichodesmium Fe limitation of N2 fixation and high isiB expression in prior EDTA-buffered culture experiments (Chappell and Webb, 2010), which is significant because Fe complexed to the synthetic ligand EDTA is not available to phytoplankton (Anderson et al., 1978). Therefore, in EDTA-buffered cultures the concentration of the small inorganic Fe fraction (predominantly Fe-hydroxy complexes) is the bioavailable form. Characterization of organic complexation in the samples collected on cruise KM0701, indicated that the [Fe'] was ⩽1 pmol l−1 (Chappell, 2009), several orders of magnitude below the [Fe'] threshold associated with Fe limitation and isiB expression in the laboratory (Chappell and Webb, 2010). The similarity between the thresholds (dFe in the field and Fe' in EDTA-buffered cultures) and high isiB gene expression suggest that most of the naturally occurring Fe complexes in sea water are bioavailable to Trichodesmium colonies, even though Fe complexes with EDTA are not, a conclusion that is supported by the consistency of our data with past models (Krishnamurthy et al., 2007), noted above.
Many bacteria use siderophores (Fe-chelating organic molecules) to assist in Fe acquisition (Andrews et al., 2003). No obvious siderophore biosynthetic genes have been identified in the Synechococcus, Prochlorococcus and Trichodesmium genomes (Rivers et al., 2009; Chappell and Webb, 2010). Crocosphaera watsonii WH8501 does have a large number of polyketide synthases and nonribosomal peptide synthetase genes indicative of secondary metabolite production (Ehrenreich et al., 2005), but none of these genes have been implicated in siderophore production. These data imply that open ocean cyanobacteria might use fundamentally different mechanism(s) to acquire organically complexed Fe. There is some genomic evidence for Trichodesmium acquiring siderophore-bound Fe using a TonB-ExbBD protein complex, although the outer membrane Fe/siderophore receptors required for high-affinity transport have not been identified (Chappell and Webb, 2010). There are also field data showing that siderophore-bound Fe can be available to Trichodesmium colonies (Achilles et al., 2003). Other potential mechanisms for Fe acquisition include the photochemical release of Fe from ligands (Barbeau et al., 2003), cell-surface reduction of ligand-bound Fe (Maldonado and Price, 2001), and interactions between Trichodesmium and the microbial consortium associated with its colonies. Fitting with our data, others have also recently shown that ferrihydrite Fe contained in dust is rapidly dissolved and rendered bioavailable by Trichodesmium colonies (Rubin et al., 2011); however, the mechanism used is currently undefined. Regardless of the uncertainty associated with the cellular mechanisms that Trichodesmium employs for Fe acquisition (either dissolved or mineral), our data suggest that Trichodesmium is able to access most if not all of the Fe in the total, dissolved pool of Fe in the upper oligotrophic ocean including Fe that is bound to organic ligands.
Select measurements of N2 fixation in Trichodesmium colonies obtained from different Fe regimes verified that high isiB expression correlated with low N2-fixation rates (Figure 5). These data corroborate our laboratory study (Chappell and Webb, 2010) and show that isiB expression is a valid marker for Fe limitation of in situ Trichodesmium N2 fixation. Frequently, the scarcity of Trichodesmium biomass in low Fe regimes precludes the implementation of rate measurements that would test for Fe limitation of N2 fixation, whereas our isiB expression method, which requires less material, can still probe the in situ Fe status of Trichodesmium in these low-biomass regimes. We are unable to directly comment on the validity of using isiB as an in situ marker for growth limitation of Trichodesmium as we did not measure C fixation on the cruises and culture data show that increases in isiB expression and decreases in N2 fixation can occur before Trichodesmium growth rates decrease significantly (Chappell and Webb, 2010). However, the isiB expression threshold level chosen for this field study was only observed in growth-limited laboratory cultures (Chappell and Webb, 2010), which suggests that Trichodesmium in situ growth was also limited by Fe in the Pacific Ocean stations (Figure 4).
Because of the direct role of Trichodesmium in CO2 and N2 fixation and its indirect role in providing ‘new' N to other phytoplankton, Trichodesmium is a biogeochemically essential organism (Capone et al., 1997); thus, defining where and when Trichodesmium activity is reduced or enhanced is important for current estimates and future forecasts of both the global C and N cycles. By comparing our Trichodesmium-specific isiB expression data with chemical measurements, this study provides empirically tested thresholds for predicting where Fe is the nutrient limiting Trichodesmium N2 fixation when only the dFe/DIP ratio or dFe are available. Furthermore, as our data imply that all of the dissolved Fe forms found in the open ocean are available to Trichodesmium colonies, extrapolation of thresholds based on total dFe to other oceanic regimes is much simplified. Such extrapolation can explain why Trichodesmium spp. are so abundant in the Western North Atlantic (Capone et al., 2005) and the Australian Shelf–Coral Sea (Capone et al., 1997), while being almost completely absent in the South Atlantic (Moore et al., 2009) and South Pacific Gyre (Bonnet et al., 2008).
Acknowledgments
This work was supported by National Science Foundation Grants OCE-0623499 and OCE-0220945. Funding for PDC was supplied by the Woods Hole Oceanographic Institution Academic Programs Office, the Center for Environmental Bioinorganic Chemistry at Princeton University, and a National Defense Science and Engineering Graduate Fellowship. We thank the captain and crew of the R/V Oceanus cruise OCE399-4 and chief scientists J Zehr and J Montoya, the captain and crew of the R/V Seward Johnson cruise SJ0609 and chief scientists Z Johnson and E Zinser and the captain and crew of the R/V Kilo Moana cruise KM0701. We thank T Goepfert, R Jakuba, W Krey, D Ohnemus, A Rose, B Wilson and F Valois for assistance with sample collection and technical support in the laboratory. We also thank E Boyle, D Capone, S Doney, J Sohm and J Waterbury for reviewing an earlier version of this manuscript.
Author contributions
EAW, JWM and PDC conceived of the study, and PDC, EAW, JWM and AMH performed the research and wrote the manuscript.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Achilles KM, Church TM, Wilhelm SW, Luther GW, Hutchins DA. Bioavailability of iron to Trichodesmium colonies in the western subtropical Atlantic Ocean. Limnol Oceanogr. 2003;48:2250–2255. [Google Scholar]
- Anderson MA, Morel FMM, Guillard RRL. Growth limitation of a coastal diatom by low zinc iron activity. Nature. 1978;276:70–71. [Google Scholar]
- Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- Barbeau K, Rue EL, Trick CG, Bruland KT, Butler A. Photochemical reactivity of siderophores produced by marine heterotrophic bacteria and cyanobacteria based on characteristic Fe(III) binding groups. Limnol Oceanogr. 2003;48:1069–1078. [Google Scholar]
- Batchelli S, Muller FLL, Chang KC, Lee CL. Evidence for strong but dynamic iron-humic colloidal associations in humic-rich coastal waters. Environ Sci Technol. 2010;44:8485–8490. doi: 10.1021/es101081c. [DOI] [PubMed] [Google Scholar]
- Benporath J, Carpenter EJ, Zehr J. Genotypic relationships in Trichodesmium (Cyanophyceae) based on nifH sequence comparisons. J Phycol. 1993;29:806–810. [Google Scholar]
- Berman-Frank I, Cullen JT, Shaked Y, Sherrell RM, Falkowski PG. Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol Oceanogr. 2001;46:1249–1260. [Google Scholar]
- Berman-Frank I, Quigg A, Finkel ZV, Irwin AJ, Haramaty L. Nitrogen-fixation strategies and Fe requirements in cyanobacteria. Limnol Oceanogr. 2007;52:2260–2269. [Google Scholar]
- Bonnet S, Guieu C, Bruyant F, Prasil O, Van WF, Raimbault P, et al. Nutrient limitation of primary productivity in the Southeast Pacific (BIOSOPE cruise) Biogeosciences. 2008;5:215–225. [Google Scholar]
- Breitbarth E, Oschilies A, LaRoche J. Physiological constraints on the global distribution of Trichodesmium - effect of temperature on diazotrophy. Biogeosciences. 2007;4:53–61. [Google Scholar]
- Breitbarth E, Wohlers J, Klas J, LaRoche J, Peeken I. Nitrogen fixation and growth rates of Trichodesmium IMS-101 as a function of light intensity. Mar Ecol Prog Ser. 2008;359:25–36. [Google Scholar]
- Capone DG.1993Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedureIn: Kemp PF, Sherr BF, Sherr EB, Cole J (eds).Handbook of Methods in Aquatic Microbial Ecology Lewis Publishers: Boca Raton, FL; 621–631. [Google Scholar]
- Capone DG, Burns JA, Montoya JP, Subramaniam A, Mahaffey C, Gunderson T, et al. Nitrogen fixation by Trichodesmium spp.: an important source of new nitrogen to the tropical and subtropical North Atlantic Ocean. Global Biogeochem Cycles. 2005;19:GB2024. [Google Scholar]
- Capone DG, Zehr JP, Paerl HW, Bergman B, Carpenter EJ. Trichodesmium, a globally significant marine cyanobacterium. Science. 1997;276:1221–1229. [Google Scholar]
- Carpenter EJ, O'Neil JM, Dawson R, Capone DG, Siddiqui PJA, Roenneberg T, et al. The tropical diazotrophic phytoplankter Trichodesmium: biological characteristics of two common species. Mar Ecol Prog Ser. 1993;95:295–304. [Google Scholar]
- Carpenter EJ, Price CC. Nitrogen fixation, distribution and production of Oscillatoria (Trichodesmium) spp. in the Western Sargasso and Caribbean Seas. Limnol Oceanogr. 1977;22:60–72. [Google Scholar]
- Chappell PD.2009. Iron and nitrogen fixation in Trichodesmium spp. MIT/WHOI Joint Program PhD thesis, Cambridge, MA [Google Scholar]
- Chappell PD, Webb EA. A molecular assessment of the iron stress response in the two phylogenetic clades of Trichodesmium. Environ Microbiol. 2010;12:13–27. doi: 10.1111/j.1462-2920.2009.02026.x. [DOI] [PubMed] [Google Scholar]
- Coles VJ, Hood RR. Modeling the impact of iron and phosphorus limitations on nitrogen fixation in the Atlantic Ocean. Biogeosciences. 2007;4:455–479. [Google Scholar]
- Cutter GA, Andersson P, Codispoti L, Croot PL, Francois R, Lohan M, et al. 2010Sampling and sample-handling protocols for GEOTRACES cruises . http://www.jodc.go.jp/geotraces/docs/GEOTRACES_IPYProtocols-Final.pdf .
- Dyhrman ST, Chappell PD, Haley ST, Moffett JW, Orchard ED, Waterbury JB, et al. Phosphonate utilization by the globally important marine diazotroph Trichodesmium. Nature. 2006;439:68–71. doi: 10.1038/nature04203. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich IM, Waterbury JB, Webb EA. Distribution and diversity of natural product genes in marine and freshwater cyanobacterial cultures and genomes. Appl Environ Microbiol. 2005;71:7401–7413. doi: 10.1128/AEM.71.11.7401-7413.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski PG. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387:272–275. [Google Scholar]
- Fu FX, Bell PRF. Growth, N2 fixation and photosynthesis in a cyanobacterium, Trichodesmium sp., under Fe stress. Biotechnol Lett. 2003;25:645–649. doi: 10.1023/a:1023068232375. [DOI] [PubMed] [Google Scholar]
- Gruber N, Sarmiento JL. Global patterns of marine nitrogen fixation and denitrification. Global Biogeochem Cycles. 1997;11:235–266. [Google Scholar]
- Hassler CS, Twiss MR. Bioavailability of iron sensed by a phytoplanktonic Fe-bioreporter. Environ Sci Technol. 2006;40:2544–2551. doi: 10.1021/es051795i. [DOI] [PubMed] [Google Scholar]
- Herbland A, Lebouteiller A, Raimbault P. Size structure of phytoplankton biomass in the equatorial Atlantic Ocean. Deep-Sea Res. 1985;32:819–836. [Google Scholar]
- Hudson RJM, Morel FMM. Trace-metal transport by marine microorganisms - implications of metal coordination kinetics. Deep-Sea Res (1 Oceanogr Res Pap) 1993;40:129–150. [Google Scholar]
- Hutchins DA, Fu FX, Zhang Y. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: implications for past, present, and future ocean biogeochemistry. Limnol Oceanogr. 2007;52:1293–1304. [Google Scholar]
- Hynes AM.2009. Diversity of the marine Cyanobacterium Trichodesmium: characterization of the Woods Hole culture collection and quantification of field populations. MIT/WHOI Joint Program PhD thesis, Cambridge, MA [Google Scholar]
- Hynes AM, Chappell PD, Dyhrman ST, Doney SC, Webb EA. Cross-basin comparison of phosphorus stress and nitrogen fixation in Trichodesmium. Limnol Oceanogr. 2009;54:1438–1448. [Google Scholar]
- Hynes AM, Webb EA, Doney SC, Waterbury JB. Comparison of cultured Trichodesmium (Cyanophyceae) with species characterized from the field. J Phycol. 2012;48:196–210. doi: 10.1111/j.1529-8817.2011.01096.x. [DOI] [PubMed] [Google Scholar]
- Jakuba RW, Moffett JW, Dyhrman ST.2008Evidence for the linked biogeochemical cycling of zinc, cobalt, and phosphorus in the western North Atlantic Ocean Global Biogeochem Cycles 22ARTN GB4012. [Google Scholar]
- Janson S, Bergman B, Carpenter EJ, Giovannoni SJ, Vergin K. Genetic analysis of natural populations of the marine diazotrophic cyanobacterium Trichodesmium. FEMS Microbiol Ecol. 1999;30:57–65. [Google Scholar]
- Kolber ZS, Barber RT, Coale KH, Fitzwater SE, Greene RM, Johnson KS, et al. Iron limitation of phytoplankton photosynthesis in the Equatorial Pacific Ocean. Nature. 1994;371:145–149. [Google Scholar]
- Krishnamurthy A, Moore JK, Zender CS, Luo C.2007Effects of atmospheric inorganic nitrogen deposition on ocean biogeochemistry J Geophys Res 112ARTN G02019. [Google Scholar]
- Kupper H, Setlik I, Seibert S, Prasil O, Setlikova E, Strittmatter M, et al. Iron limitation in the marine cyanobacterium Trichodesmium reveals new insights into regulation of photosynthesis and nitrogen fixation. New Phytol. 2008;179:784–798. doi: 10.1111/j.1469-8137.2008.02497.x. [DOI] [PubMed] [Google Scholar]
- Kustka A, Sanudo-Wilhelmy S, Carpenter EJ, Capone DG, Raven JA. A revised estimate of the iron use efficiency of nitrogen fixation, with special reference to the marine cyanobacterium Trichodesmium spp. (Cyanophyta) J Phycol. 2003a;39:12–25. [Google Scholar]
- Kustka AB, Sanudo-Wilhelmy SA, Carpenter EJ, Capone D, Burns J, Sunda WG. Iron requirements for dinitrogen- and ammonium-supported growth in cultures of Trichodesmium (IMS 101): Comparison with nitrogen fixation rates and iron: carbon ratios of field populations. Limnol Oceanogr. 2003b;48:1869–1884. [Google Scholar]
- LaRoche J, Boyd PW, McKay RML, Geider RJ. Flavodoxin as an in situ marker for iron stress in phytoplankton. Nature. 1996;382:802–805. [Google Scholar]
- Levitan O, Rosenberg G, Setlik I, Setlikova E, Grigel J, Klepetar J, et al. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Global Change Biol. 2007;13:531–538. [Google Scholar]
- Lundgren P, Janson S, Jonasson S, Singer A, Bergman B. Unveiling of novel radiations within Trichodesmium cluster by hetR gene sequence analysis. Appl Environ Microbiol. 2005;71:190–196. doi: 10.1128/AEM.71.1.190-196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahowald NM, Engelstaedter S, Luo C, Sealy A, Artaxo P, Benitez-Nelson C, et al. Atmospheric iron deposition: global distribution, variability, and human perturbations. Annu Rev Mar Sci. 2009;1:245–278. doi: 10.1146/annurev.marine.010908.163727. [DOI] [PubMed] [Google Scholar]
- Maldonado MT, Price NM. Reduction and transport of organically bound iron by Thalassiosira oceanica (Bacillariophyceae) J Phycol. 2001;37:298–309. [Google Scholar]
- Mills MM, Ridame C, Davey M, La Roche J, Geider RJ. Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature. 2004;429:292–294. doi: 10.1038/nature02550. [DOI] [PubMed] [Google Scholar]
- Moore CM, Mills MM, Achterberg EP, Geider RJ, La Roche J, Lucas MI, et al. Large-scale distribution of Atlantic nitrogen fixation controlled by iron availability. Nature Geosci. 2009;2:867–871. [Google Scholar]
- Moore JK, Doney SC.2007Iron availability limits the ocean nitrogen inventory stabilizing feedbacks between marine denitrification and nitrogen fixation Global Biogeochem Cycles 21ARTN GB2001. [Google Scholar]
- Moore JK, Doney SC, Lindsay K.2004Upper ocean ecosystem dynamics and iron cycling in a global three-dimensional model Global Biogeochem Cycles 18ARTN GB4028. [Google Scholar]
- Moutin T, Van Den Broeck N, Beker B, Dupouy C, Rimmelin P, Le Bouteiller A. Phosphate availability controls Trichodesmium spp. biomass in the SW Pacific Ocean. Mar Ecol Prog Ser. 2005;297:15–21. [Google Scholar]
- Orcutt KM, Rasmussen U, Webb EA, Waterbury JB, Gundersen K, Bergman B. Characterization of Trichodesmium spp. by genetic techniques. Appl Environ Microbiol. 2002;68:2236–2245. doi: 10.1128/AEM.68.5.2236-2245.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JBe, Biswas H, Schulz KG, Laroche J, Riebesell U. Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium. Global Biogeochem Cycles. 2007;21:GB2028. [Google Scholar]
- Rivers AR, Jakuba RW, Webb EA. Iron stress genes in marine Synechococcus and the development of a flow cytometric iron stress assay. Environ Microbiol. 2009;11:382–396. doi: 10.1111/j.1462-2920.2008.01778.x. [DOI] [PubMed] [Google Scholar]
- Rubin M, Berman-Frank I, Shaked Y. Dust- and mineral-iron utilization by the marine dinitrogen-fixer Trichodesmium. Nat Geosci. 2011;4:529–534. [Google Scholar]
- Rue EL, Bruland KW. Complexation of iron(III) by natural organic ligands in the Central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar Chem. 1995;50:117–138. [Google Scholar]
- Saito MA, Schneider DL. Examination of precipitation chemistry and improvements in precision using the Mg(OH)2 preconcentration inductively coupled plasma mass spectrometry (ICP-MS) method for high-throughput analysis of open-ocean Fe and Mn in seawater. Anal Chim Acta. 2006;565:222–233. [Google Scholar]
- Sanudo-Wilhelmy SA, Kustka AB, Gobler CJ, Hutchins DA, Yang M, Lwiza K, et al. Phosphorus limitation of nitrogen fixation by Trichodesmium in the central Atlantic Ocean. Nature. 2001;411:66–69. doi: 10.1038/35075041. [DOI] [PubMed] [Google Scholar]
- Shi T, Sun Y, Falkowski PG. Effects of iron limitation on the expression of metabolic genes in the marine cyanobacterium Trichodesmium erythraeum IMS 101. Environ Microbiol. 2007;9:2945–2956. doi: 10.1111/j.1462-2920.2007.01406.x. [DOI] [PubMed] [Google Scholar]
- Sohm JA, Mahaffey C, Capone DG. Assessment of relative phosphorus limitation of Trichodesmium spp. in the North Pacific, North Atlantic, and the north coast of Australia. Limnol Oceanogr. 2008;53:2495–2502. [Google Scholar]
- Webb EA, Jakuba RW, Moffett JW, Dyhrman ST. Molecular assessment of phosphorus and iron physiology in Trichodesmium populations from the western Central and western South Atlantic. Limnol Oceanogr. 2007;52:2221–2232. [Google Scholar]
- Webb EA, Moffett JW, Waterbury JB. Iron stress in open-ocean cyanobacteria (Synechococcus, Trichodesmium, and Crocosphaera spp.): identification of the IdiA protein. Appl Environ Microbiol. 2001;67:5444–5452. doi: 10.1128/AEM.67.12.5444-5452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Boyle E, Sunda W, Wen L. Soluble and colloidal iron in the oligotrophic North Atlantic and North Pacific. Science. 2001;293:847–849. doi: 10.1126/science.1059251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.