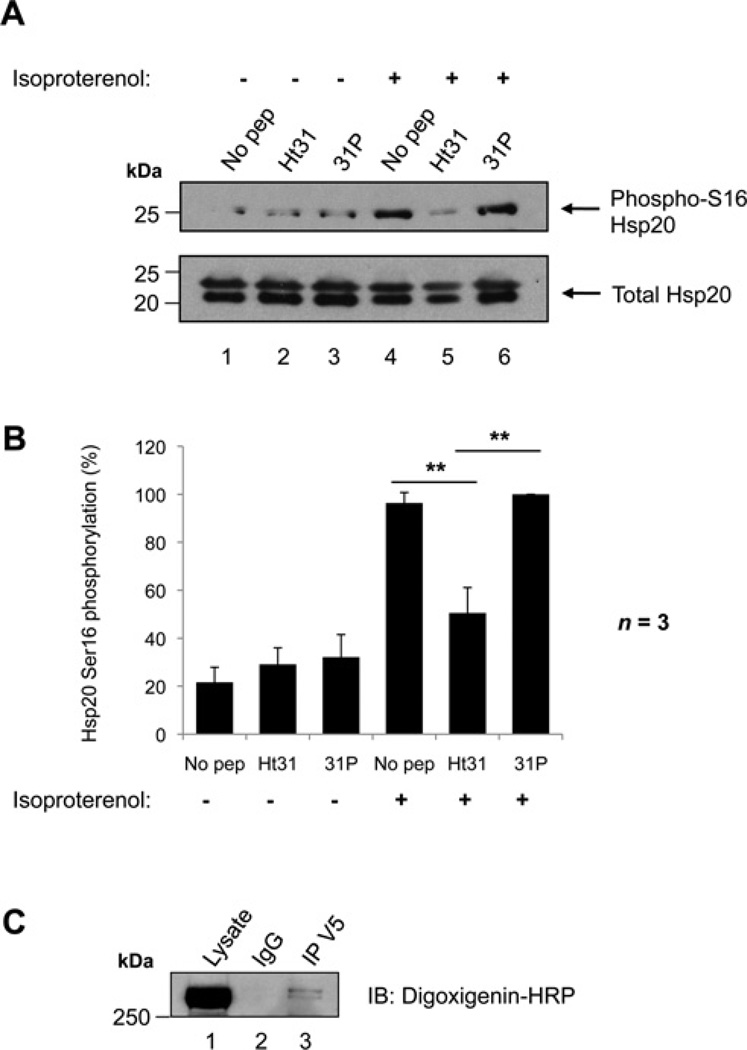
Figure 1. Phosphorylation of Hsp20 on Ser16 is facilitated by an AKAP.
(A) Ht31 treatment of NRVMs. Cells were treated with either 50 µM Ht31 peptide, Ht31P control peptide, or no peptide as indicated for 30 min, and then immediately harvested (lanes 1–3) or stimulated with 1 nM isoproterenol for 5 min prior to harvesting (lanes 4–6) to activate PKA and promote Hsp20-Ser16 phosphorylation. Cellular lysates were separated by SDS/PAGE and transferred on to nitrocellulose. Membranes were probed with anti-(phospho-Ser16 Hsp20) (upper panel) or anti-(total Hsp20) antibodies (lower panel). Immunoblots are representative of three independent experiments. (B) Quantification of data in (A). Bands were quantified using NIH ImageJ software. The amount of phospho-Ser16 Hsp20 was normalized to the amount of total Hsp20 (detected as a doublet in these cells), and is expressed as a percentage of the maximum phosphorylation measured. The results are the means ± S.E.M. of three independent experiments. **P < 0.01 indicates a highly significant difference between Ht31 peptide treatment compared with no peptide treatment, and Ht31 peptide treatment compared with 31P control peptide treatment. (C) RII overlay of an Hsp20 immunoprecipitation. Hsp20–V5 was transfected in to HEK-293 cells. Cellular lysates were immunoprecipitated with anti-V5 antibody or isotype-matched IgG, separated by SDS/PAGE and transferred on to nitrocellulose. Immunoprecipitates were probed with digoxigenin-labelled PKA RII subunits followed by anti-digoxigenin–HRP to detect RII-binding proteins. Molecular masses in kDa are indicated to the left-hand side of Western blots.