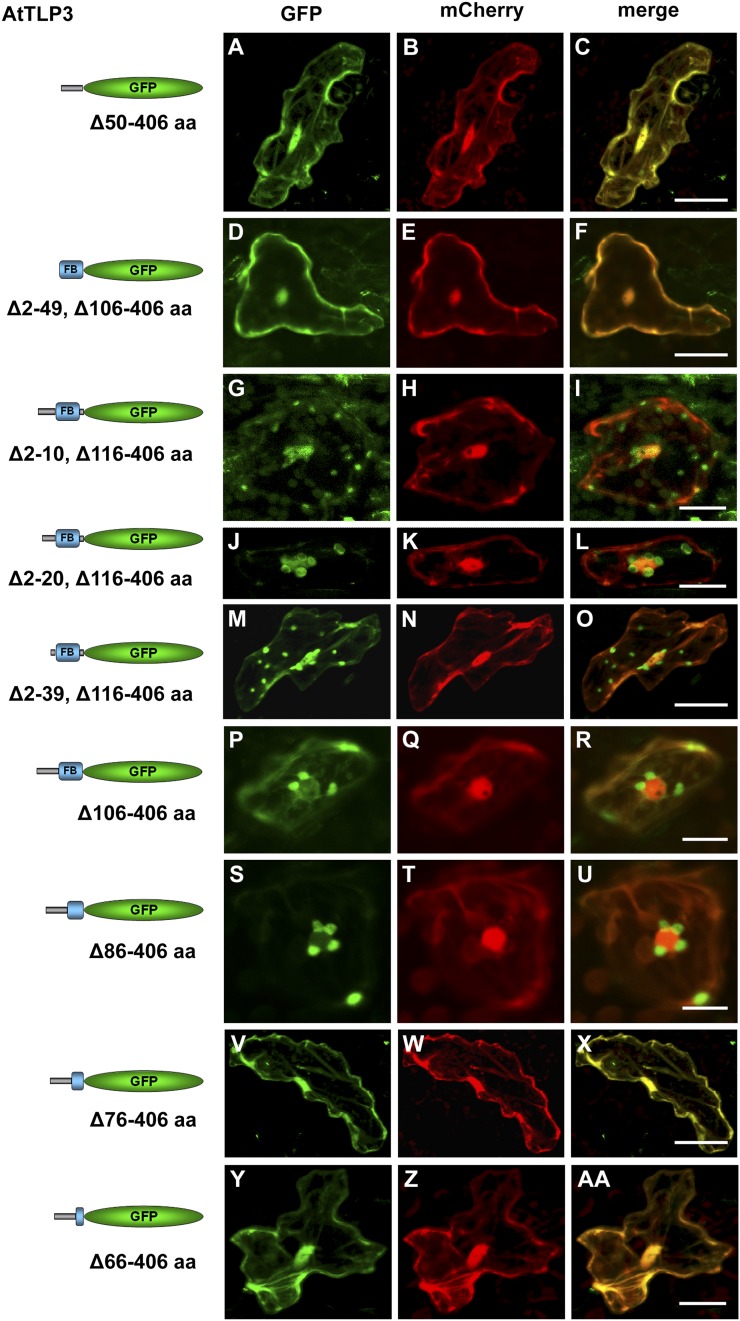
Figure 5.
Parts of the leading sequence and FB domain determine plastidial localization of the N terminus of AtTLP3. A, D, G, J, M, P, S, V, and Y, Arabidopsis leaf cells were transiently transformed with truncated versions of the N terminus of AtTLP3 fused to GFP. Constructs used for transformation are indicated on the left. B, E, H, K, N, Q, T, W, and Z, Leaf cells were cotransformed with the cytosolic and nucleoplasmic marker mCherry. C, F, I, L, O, R, U, X, and AA, Merged images indicate the subcellular localization of truncated versions. Yellow color indicates the colocalization of green- and red-fluorescing proteins. All truncated versions lack amino acids (aa) of the C terminus (Tubby domain; amino acids 116–406) of AtTLP3, while the linker sequence (amino acids 106–115) is either missing (Δ116–406) or included (Δ106–406). In addition, various amino acids of the N terminus are missing as indicated. A to F, Cytosolic and nucleoplasmic localization of GFP fused to the leading sequence (Δ50–406; A–C) or the FB domain (Δ2–49, Δ106–406; D–F). G to O, Plastidial and nucleocytosolic localization of GFP fused to truncated N-terminal versions lacking amino acids 2 to 10 (Δ2–10, Δ106–406; G–I), 2 to 20 (Δ2–20, Δ106–406; J–l), or 2 to 39 (Δ2–39, Δ106–406; M–O). P to R, Plastidial localization of GFP fused to an N-terminal version lacking the linker sequence and Tubby domain (Δ106–406). S to U, Plastidial localization of GFP fused to an N-terminal version lacking the linker sequence, Tubby domain, and the last 20 amino acids of the FB (Δ86–406). V to AA, Cytosolic and nucleoplasmic localization of GFP fused to an N-terminal version lacking the linker sequence, Tubby domain, and the last 30 (Δ76–406; V–X) or 40 (Δ66–406; Y–AA) amino acids of the FB. Experiments were repeated three times with similar results. Bars = 20 µm.