Table 2.
Synthesis and in situ use of arylaluminum reagents in Cu-catalyzed enantioselective allylic substitution reactions.[a]
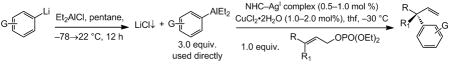
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Aryllithium | Substrate [R; R1] | NHC–AgI; mol % | t (h) | SN2′:SN2[b] | Yield [%][c] | e.r.[d] |
| 1 | PhLi | CO2tBu; Me | 2a; 0.5 | 0.5 | >98:2 | 98 | 91:9 |
| 2 | PhLi | CO2tBu; Et | 2a; 0.5 | 3.0 | >98:2 | 96 | 91.5:8.5 |
| 3 | pOMeC6H4Li | CO2tBu; Me | 2a; 0.5 | 1.0 | >98:2 | 87 | 90.5:9.5 |
| 4 | pCF3C6H4Li | CO2tBu; Me | 1; 0.75 | 1.5 | >98:2 | 88 | 83:17 |
| 5 | PhLi | SiMe2Ph; Me | 2a; 1.0 | 3.0 | >98:2 | 81 | 96:4 |
| 6 | pOMeC6H4Li | SiMe2Ph; Me | 2a; 1.0 | 3.0 | >98:2 | 97 | 97:3 |
Reactions were performed under N2 atmosphere; >98% conversion in all cases.
Determined through analysis of 400 MHz 1H NMR spectra of unpurified mixtures.
Yields of isolated purified products.
Determined by GLC or HPLC analysis; see the Supporting Information for details.
