Table 3.
Synthesis and in situ use of heterocyclic aluminum reagents in Cu-catalyzed enantioselective allylic substitution reactions.[a]
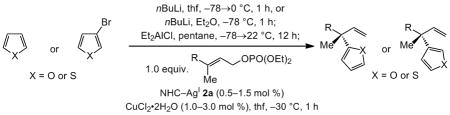
| ||||||
|---|---|---|---|---|---|---|
| Entry | Heterocycle | Substrate [R] | mol % 2a | SN2′:SN2[b] | Yield [%][c] | e.r.[d] |
| 1 | 2-furyl | Ph | 0.5 | >98:2 | 93 | >98:2 |
| 2 | 2-furyl | oBrC6H4 | 0.5 | >98:2 | 86 | >98:2 |
| 3 | 2-furyl | oMeOC6H4 | 0.5 | >98:2 | 95 | >98:2 |
| 4 | 2-furyl | oMeC6H4 | 1.0 | >98:2 | 98 | 98:2 |
| 5 | 2-furyl | pNO2C6H4 | 0.5 | >98:2 | 96 | >98:2 |
| 6 | 2-furyl | SiMe2Ph | 1.0 | >98:2 | 91 | 86.5:13.5 |
| 7[e] | 3-furyl | Ph | 1.0 | >98:2 | 90 | 97:3 |
| 8 | 2-thienyl | Ph | 0.5 | >98:2 | 98 | 96:4 |
| 9 | 2-thienyl | oBrC6H4 | 1.5 | >98:2 | 98 | 98:2 |
| 10 | 2-thienyl | pNO2C6H4 | 0.5 | >98:2 | 96 | 94:6 |
| 11 | 2-thienyl | CO2tBu | 0.5 | >98:2 | 94 | 87.5:12.5 |
| 12[e] | 3-thienyl | Ph | 1.0 | >98:2 | 94 | 94:6 |
| 13[e] | 3-thienyl | oBrC6H4 | 1.0 | >98:2 | 89 | 97:3 |
| 14[e] | 3-thienyl | SiMe2Ph | 1.0 | >98:2 | 95 | 94:6 |
See Table 2.
The corresponding 3-bromofuran or 3-bromothiophene used as starting materials (treatment with nBuLi in Et2O); see the Supporting Information for experimental details.
