Abstract
Fluent readers process written text rapidly and accurately, and comprehend what they read. Historically, reading fluency has been modeled as the product of discrete skills such as single word decoding. More recent conceptualizations emphasize that fluent reading is the product of competency in, and the coordination of, multiple cognitive sub‐skills (a multi‐componential view). In this study, we examined how the pattern of activation in core reading regions changes as the ability to read fluently is manipulated through reading speed. We evaluated 13 right‐handed adults with a novel fMRI task assessing fluent sentence reading and lower‐order letter reading at each participant's normal fluent reading speed, as well as constrained (slowed) and accelerated reading speeds. Comparing fluent reading conditions with rest revealed regions including bilateral occipito‐fusiform, left middle temporal, and inferior frontal gyral clusters across reading speeds. The selectivity of these regions' responses to fluent sentence reading was shown by comparison with the letter reading task. Region of interest analyses showed that at constrained and accelerated speeds these regions responded significantly more to fluent sentence reading. Critically, as reading speed increased, activation increased in a single reading‐related region: occipital/fusiform cortex (left > right). These results demonstrate that while brain regions engaged in reading respond selectively during fluent reading, these regions respond differently as the ability to read fluently is manipulated. Implications for our understanding of reading fluency, reading development, and reading disorders are discussed. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: reading, language, fluency, fluent, fMRI, event‐related
INTRODUCTION
Fluent reading occurs when individuals read rapidly and accurately while comprehending what is read [Lyon et al., 2003; National Reading Panel (NRP), 2000]. Recently, models of reading fluency have emphasized the multiple contributions of reading‐related components, such as orthographic, phonological, syntactic, and semantic processing [e.g., Kame'enui and Simmons, 2001; Katzir et al., 2006; Wolf and Katzir‐Cohen, 2001], rather than conceptualizing fluency as the outcome of discrete skills such as word identification. In this study, we designed a functional imaging assessment of reading fluency to understand whether variation in the ability to read fluently, achieved through varying reading speed, altered activation through key brain regions engaged during reading in a “componential” manner consistent with this new view of fluency.
In the field of reading research, historic views of fluency have tended to be somewhat narrow and functional, with the significant benefit of allowing a relatively straightforward assessment of fluency in (for instance] a classroom setting [e.g., Hasbrouck and Tindal, 1992]. Such definitions may conceptualize fluency as, for example, speed and accuracy in oral reading [Hasbrouck and Tindal, 1992; Shinn et al., 1992]. This view was described by Torgesen et al. [2001], who noted its benefits in an assessment setting and in describing key differences between reading disabled readers and typical controls. While this view does describe the output of a fluent reading system, it does not acknowledge the role played by the multiple lower‐order sub‐skills fluent reading requires. For example, in their review of relevant literature, the NRP [2000] concluded that fluency follows efficient word recognition—a view neglecting such component sub‐skills as semantic processing. Historically, such a definition has been used to make important decisions relating to eligibility for and the effectiveness of special education programs [see discussion in Shinn et al., 1992].
More recent conceptualizations depict reading fluency as the product of each reading sub‐skill, as well as the integration of these skills [e.g., Kame'enui and Simmons, 2001; Wolf and Katzir‐Cohen, 2001]. Such conceptualizations emphasize the achievement of proficiency in and automatization of all lower‐order reading skills to allow fluent reading. Here fluency can be considered a property of key cognitive processes (e.g., orthographic, phonologic, and semantic processing), as well as lower‐order perceptual, higher attentive, and potentially executive skills [Berninger et al., 2001; Wolf and Katzir‐Cohen, 2001].
Significant behavioral evidence supports this view of fluency as being componential in nature. The likelihood that reading difficulties can result from multiple, differing core deficits has been considered for some time [e.g., Seymour, 1987] and is currently a prominent view [e.g., Katzir et al., 2008; Wolf et al., 2000a]. Fluency deficits form a core feature of reading disorders [e.g., British Psychological Society, 1999; Lyon et al., 2003] that are not necessarily remediated by interventions targeting lower‐order reading skills [e.g., phonemic awareness; see Lyon and Moats, 1997]. Further, the development of fluency in lower‐level reading sub‐skills (word level recognition skill) does not necessarily predict connected text fluency [e.g., NRP, 2000], and multiple behavioral studies have now focused on higher‐level connected text fluency [e.g., Kame'enui et al., 2001; Katzir et al., 2006, 2008; see discussion in Katzir et al., 2006]. For example, Katzir et al. [2006] found evidence for distinct reading sub‐skills (orthography, phonological processing, and rapid naming) predicting fluency at the word reading fluency level, and different elements of fluency (reading rate, accuracy, and comprehension) at the connected text level. Katzir et al. [2008] have shown that children with deficits in different lower‐order reading components (e.g., phonological processing and rapid naming) experience deficits at different levels of reading fluency.
In this study, we used functional MRI (fMRI) to characterize the relationship between reading fluency and the brain regions supporting reading sub‐skills. The nature of this relationship has important implications. If reading fluency is indexed by the engagement of multiple reading sub‐skills, it should be considered from the earliest stages of reading development and education; methods remediating fluency deficits may best target separate reading sub‐skills; and individuals with a single (behavioral) fluency deficit may be further characterized or even subtyped according to (the lack of) activation in neural structures supporting one or more reading sub‐skills. Further, if reading fluency is better characterized as a property of a number of reading sub‐skills, the implications for fluency instruction may differ; e.g., fluency interventions may best target all component sub‐skills rather than, for instance, decoding skills in isolation.
Neural Structures Supporting Reading
Broadly, distinct posterior and anterior neural systems are engaged in reading [e.g., Pugh et al., 2000, 2001; Shaywitz et al., 2006]. The posterior reading networks include a ventral (notably left fusiform) component involved in orthographic processes and more dorsal temporo‐parietal regions (e.g., superior temporal lobe, angular/supramarginal gyri) involved in the phonemic processing and phoneme‐grapheme mapping [see Shaywitz and Shaywitz, 2008 for a detailed overview]. The more anterior network, including the left inferior frontal gyrus, is involved in higher‐order processing (e.g., semantics, comprehension, and grammatical elements). These networks coordinate and adapt through reading development in a process referred to as “interactive specialization” [e.g., Schlaggar and McCandliss, 2007]. Individuals who experience reading difficulties tend to under‐activate the posterior systems and over‐activate the anterior network [Hoeft et al., 2006; Shaywitz and Shaywitz, 2008; Shaywitz et al., 2006; Temple, 2002].
Functional imaging evidence for these networks has largely come from examination of reading's component processes [e.g., Baker et al., 2007; Booth et al., 2002a, b; Brem et al., 2006; Petersen et al., 1988; Simos et al., 2001; van Atteveldt et al., 2004]. A number of studies have also examined engagement of anterior and posterior networks during higher‐order sentence comprehension, during which neural activation differs from that during lower‐order processes largely in terms of the extent of activation [e.g., Cooke et al., 2006; Jobard et al., 2007; see Rimrodt et al., 2009 for discussion]. Researchers have also studied participants engaged in fluent reading to examine reading‐related constructs such as lexicality [e.g., Yarkoni et al., 2008], sentence comprehension [e.g., Meyler et al., 2007; Rimrodt et al., 2009], and semantics [e.g., Schulz et al., 2009]. While some studies have used sentence‐reading paradigms to show differences between typical readers and individuals with dyslexia [e.g., Helenius et al., 1999; Kronbichler et al., 2006; Meyler et al., 2007; Rimrodt et al., 2009], few functional imaging studies have examined the correlates of reading fluency. Models conceptualizing fluency as equivalent to single word decoding processes separate from comprehension [discussed in Shinn et al., 1992] would emphasize changes in regions relating to single word decoding processes as reading fluency varied. A multi‐componential view would emphasize variation in all reading‐related structures including both those engaged in single word decoding and those engaged in sentence comprehension.
Karni et al. [2005] compared fluent reading at slow and fast speeds in small groups of typical and reading‐impaired university students. Using a blocked design, words constituting a sentence were presented one at a time in the center of a display at either a slow or fast rate. Contrasts of reading against fixation revealed activation in key brain regions implicated in reading‐including the left frontal operculum, mesial temporal gyrus, and extrastriate cortex‐at both speeds (fast apparently less so than slow). Kujala et al. [2007] used magnetoencephalography (MEG) to examine regions involved in fluent passage reading at three individually determined speeds to allow comprehension of 0, 50, or 100% of what was read (comprehension was evaluated post‐imaging). As in Karni et al., words were presented sequentially, center screen. They identified the left hemisphere reading network including inferior occipito‐temporal regions; medial, superior and anterior‐inferior temporal cortex, and pre‐central (facial motor), insula, prefrontal and orbitofrontal regions. The main network nodes were the occipitotemporal, cerebellar, and orbital regions, and as speed increased, the inferior occipital, superior temporal, and orbital network nodes became more synchronized.
Imaging Reading Fluency by Altering Reading Speed
Reading speed and accuracy are typically considered key elements of reading fluency along with (explicitly or implicitly) comprehension [e.g., NRP, 2000; Torgesen et al., 2001; Wolf and Katzir‐Cohen, 2001]. Altering the ability to read fluently through varying reading speed, while holding reading accuracy and comprehension constant, would seem to be a useful approach to imaging fluency. Such an approach entails significant methodological and cognitive challenges, however. Cognitively, word presentation speed must be set on an individual basis to equate task difficulty between participants, as a slow speed for one participant may be fast for another. Stimulus presentation style should also mirror the typical fluent reading experience as closely as possible to control cognitive processes. While words might be presented consecutively to control reading speed, ideally the words constituting the sentence would remain in view until the entire sentence was presented. By definition, participants' comprehension of sentences should also be evaluated. Methodologically, comparing fluent reading to a low‐level baseline task (e.g., rest condition) allows regions involved in all reading sub‐skills (i.e., including lower‐level perceptual processes) to be identified. An additional matched baseline (e.g., a basic letter reading task) should be included, however, so that structures selectively engaged in higher‐order fluent sentence reading can be delineated. An event‐related fMRI design also allows data from only comprehended sentences to be examined, and the different duration of events at different reading speeds to be accommodated (e.g., presenting sentences at a slower speed will take longer than those at participants' standard fluent reading speed). Finally, in any such task the number of trials in each condition, and images of task‐related activity, will vary between conditions and participants (e.g., again, there will be more for conditions where text is presented at a slower speed). If analyses are to be valid, they must accommodate the resulting differences in variance [Beckmann et al., 2003; Smith et al., 2004].
In the current study, we described the relationship between reading fluency and the brain regions supporting reading sub‐skills using fMRI to examine the pattern of neural activation as reading speed/the capacity to read fluently was systematically manipulated. Prior to scanning, healthy adult participants' normal fluent reading speed was determined. Participants then completed an event‐related fMRI task developed with reference to the above constraints, in which they fluently read sentences and letter strings at (i) their normal fluent reading speed as well as (ii) constrained, and (iii) accelerated fluent reading speeds (130% and 70% of their normal speed). We hypothesized that while fluent reading conditions would engage the structures supporting reading sub‐skills regardless of speed, activation in these structures (left fusiform, superior temporal, and inferior frontal regions) would independently vary as the ability to read fluently was altered. This would be most in line with a multi‐componential view of reading fluency [Wolf and Katzir‐Cohen, 2001].
MATERIALS AND METHODS
Participants
The study included 13 right‐handed, native English‐speaking adults (12 females) with a mean age of 24.05 years (SD 4.48 years, range 19.16–33.24 years). None had a history of reading disorder, delayed reading or language acquisition, developmental delay, or diagnosed developmental disorder. No neurological history was reported, and all participants completed the study with normal/corrected‐to‐normal vision.
Procedure
The study was approved by the Institutional Review Board at the Children's Hospital Boston and all participants provided written informed consent.
Pre‐scanning
Prior to entering the scanner the participant read, at a comfortable pace, three passages taken from the Informal Reading Inventory [Burns and Roe, 2001; second‐grade level passages] to determine their normal fluent reading speed, then answered standard comprehension questions. Each participant's average word reading speed was then calculated, along with rates 30% slower and faster. These rates were used as “normal,” “constrained,” and “accelerated” word presentation speeds in the scanner task (described below). Average word reading speeds for normal, constrained, and accelerated fluency were 239 ms (51 ms, 159–348 ms), 311 ms (66 ms, 207–452 ms), and 167 ms (36 ms, 112–243 ms) (SD, range in brackets).
In‐scanner procedure
Participants completed two 6.5‐min runs of the fluency task in the MR‐scanner. In each run, (a) fluent sentence reading and (b) letter reading tasks were alternately presented. Each of these tasks included trials completed at participants' normal, constrained, and accelerated fluent reading speeds. A participant who read at 1,000 ms per word in the pre‐scan test would complete trials in which words were presented at normal, constrained, and accelerated rates of 1,000, 1,300, and 700 ms. All trials had an identical structure (Fig. 1). An initial image cue (500 ms) indicated the following stimuli's speed. This was followed by a blank screen (200 ms), and the series of words/letter groups presented at the relevant speed. Words/control stimuli appeared sequentially from left to right at the determined rate until the complete sentence was displayed, with each word remaining on screen until all words were presented. Another blank screen followed (<1,000 ms). Finally stimuli to assess comprehension (fluent sentence reading) or text viewing (letter reading) were presented. Here three cartoon images, one showing a key element of the sentence (fluent sentence reading), or three letters, one of which was the differing letter in the sentence (letter reading), were presented. The participant then pressed one of three buttons to indicate which of the images related to the sentence (fluent sentence reading) or which of the letters differed from the others (letter reading) (3,000 ms). The location of the correct response was pseudo‐randomized in each condition and word speed. This was followed by a fixation cross, terminated by the following trial.
Figure 1.
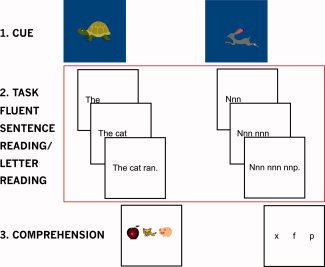
Fluent sentence reading (left) and letter reading (right) tasks. Sentence task: participants viewed a cue indicating word presentation speed (turtle = constrained; cat = normal [shown here]; rabbit = accelerated). This was followed by fluent sentence and comprehension phases. The letter reading task was identical but with groups of the letter “n” (and one differing letter) as stimuli. A crosshair was presented between trials. Red box: events modelled in analysis. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Stimuli
Sentence stimuli were taken from the Informal Reading Inventory [Burns and Roe, 2001]. Given text readability's influence on reading fluency [see Samuels, 2006], sentences were taken from paragraphs appropriate for first grade level or lower. We created two runs of stimuli, with 16 sentences per run. Overall, 11 were presented at normal and constrained speeds, 10 were accelerated. A rest condition (fixation) was presented between trials (described below). Mean sentence length was 5.03 words (SD 1.53 words, range 2–8), with minor modifications (e.g., changing the name “Paco” to “he”) and the number of words per sentence was equated between conditions and runs. Sentences were entered into the MRC database (http://www.psy.uwa.edu.au/MRCDataBase/mrc2.html), with all available key word characteristics being entered into t‐tests to examine any differences between presentation speeds or runs; none were observed (all P > 0.05). Overall, mean age of acquisition was 198 (SD 48; range 114–269) (approximately 2–4 years of age); mean Kucera–Francis written word frequency was 12,359 (22,196; 1–69,971); mean concreteness 390 (161; 180–636); mean familiarity 599 (32; 469–646); mean imagability 414 (162; 209–638); and mean number of phonemes 2.6 (1–8). Control stimuli were word‐like groups of the letter “n” matched to sentence stimuli (Fig. 1). Here, the participant's task was to read through the letters and identify the differing letter (either f, p, or x). To ensure all letters were viewed, the target letter was always presented in one of the last two words of the sentence.
Imaging Protocol and Analysis
In each imaging session two runs of 190 32‐slice (4 mm thick) functional echoplanar images were acquired (interleaved ascending acquisition) using a repetition time of 2,000 ms, echo time of 30 ms, field of view of 192 × 192 × 153 (full brain coverage), 90° flip angle and a voxel size of 3 mm × 3 mm × 4 mm. Data analysis was completed using FSL 4.1.4 (http://www.fmrib.ox.ac.uk/fsl/), with modeling completed using FEAT v5.98, and higher‐level analysis using FLAME. The first four images were discarded to accommodate field effects. Motion correction (MCFLIRT) and slice‐time correction were completed (to the slice at the mid‐point of data acquisition). Images were skull‐stripped (BET); smoothed (4 mm FWHM kernel); temporally filtered (high‐pass filter, 50s); and underwent linear registration (12 degrees of freedom) to the MNI152 T1 template (FLIRT). Registration was manually checked.
Modeling was completed in three stages. A first‐level model was structured for each session. Data were prewhitened and modeled with regressors for the (1) speed cue; (2–4) constrained, normal, and accelerated fluent sentence reading; (5–7) constrained, normal, and accelerated letter reading; (8–9) sentence and control comprehension stimuli, and (10) rest/inter‐trial fixation. Each subjects' two sessions were combined in a fixed effects model, then entered into a group random‐effects analysis (FLAME 1). Contrasts were completed using sentence and control regressors (2–7), and rest. Statistical inference was completed using Z (Gaussianized t) statistic images, thresholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P = 0.05. First level contrasts [fluent sentence reading > rest] were examined visually to confirm that subjects were not right hemisphere language dominant.
Region of interest analyses
Regions engaged in fluent reading were identified through the contrast fluent sentence reading [all conditions] > rest. Spheres (5 mm radius) were drawn around the peak activations within temporo‐occipital fusiform (FFG), middle temporal (MTG), and inferior frontal (IFG) gyri [as delineated in the Harvard‐Oxford Cortical Atlas within FSL; Smith et al., 2004; Fig. 4]. Subjects' mean contrasts of parameter estimates (COPEs) were then extracted from region of interest (ROI) for fluent sentence reading and letter reading at normal, constrained, and accelerated speeds (task > rest in each instance). Fluent sentence reading COPEs were then compared with letter reading COPEs through nine paired t‐tests (three speeds × three ROI; α of 0.05 [Bonferroni corrected = 0.006]).
Figure 4.
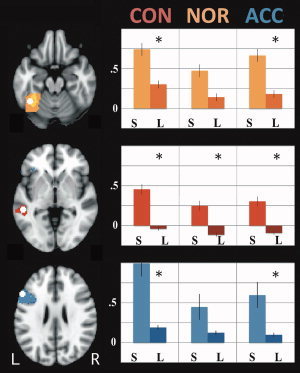
Region of interest analyses. Left: Regions (white spheres). Left fusiform (top, yellow); middle temporal (middle, red); and inferior frontal (bottom, blue) gyri. Colored areas indicate greater within‐structure activation for fluent sentence reading > rest. Right: Activation for [fluent (S)entence reading > rest] vs [(L)etter reading > rest]. CON = constrained; NOR = normal; ACC = accelerated. *Significantly different (P<0.05). Axial slices at −20 (fusiform), −2 (middle temporal), and 26 (inferior frontal). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Peri‐stimulus data extraction
Mean sentence duration (SD, range) for the normal, constrained, and accelerated conditions was 1,203 ms (257 ms, 800–1750 ms), 1,565 ms (333 ms, 1,041–2,274 ms), and 842 ms (179 ms, 563–1,222 ms) respectively. To directly examine the average blood oxygen level dependent (BOLD) response to stimuli in key contrasts, we examined data from the point of peak difference between the two conditions. Each participant had two sessions of data. We first averaged the response to trials of each condition within each session independently. The minimum signal value within each condition was then set to 0 to accommodate baseline session effects. Average BOLD signal responses were calculated for these 26 sessions of data, and values for the normal, constrained, and accelerated fluent sentence reading were finally normalized such that the values 0 and 1 reflected the minimum and maximum BOLD response for the condition in which contrasts indicated lesser activation.
RESULTS
Behavioral Data
In‐scanner performance (trial accuracy) was evaluated for both tasks. Accuracy for constrained fluent sentence reading was 99.3%; normal fluent sentence reading 99.3%; and accelerated fluent sentence reading 100%. This reflected ceiling performance across the tasks for 12 participants, with the 13th making a single error in the constrained and normal conditions. Letter reading was completed with 100% accuracy at fluent, constrained, and accelerated reading speeds.
Imaging Data
Analyses were completed in three stages. Regions engaged in letter reading and fluent sentence reading tasks were first revealed by comparing each task with a common rest condition. Whether the regions identified in the previous contrasts were selectively engaged during fluent sentence reading (as compared with letter reading) was then investigated through a ROI analysis. Finally, how altering reading speed altered activation was examined.
Regions Engaged in Letter Reading and Fluent Sentence Reading Compared to Rest
Each letter reading contrast (three contrasts; constrained, normal, accelerated > rest) revealed strong lateral occipital activation (extending anteriorly to the caudal extent of the middle temporal gyrus), postcentral gyral activity (left > right), and some bilateral precentral gyral activation in a region abutting the inferior frontal gyrus (IFG; Fig. 2, right; Table I). Furthermore, bilateral cerebellar activation was apparent as were clusters extending from the putamen to the insula.
Figure 2.
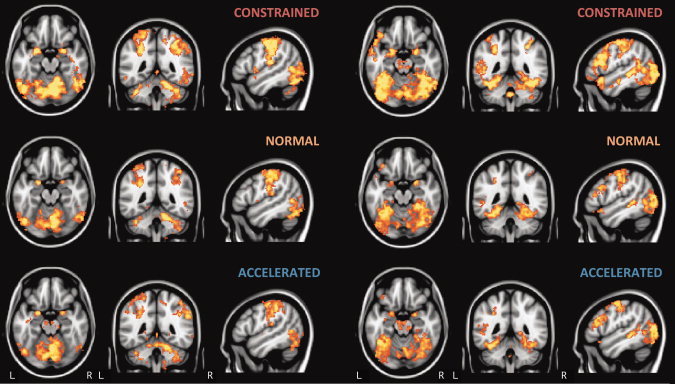
(left) Activation for letter reading tasks > rest and (right) fluent sentence reading tasks > rest by speed (constrained, normal, and accelerated). Data for Images cluster thresholded at P<0.05, z>2.3, neurological space, saggital slice through left hemisphere (MNI coordinates −50, −46, −18). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table I.
Fluent sentence reading > rest and letter reading > rest by reading speed
| Voxels | P | Z‐max | X | Y | Z | Location (Z‐max) |
|---|---|---|---|---|---|---|
| Fluent sentence reading | ||||||
| Constrained | ||||||
| 47916 | 0.000 | 4.97 | −36 | −48 | −22 | Left temporal occipital fusiform cortex |
| 4729 | 0.000 | 4.51 | 0 | −2 | 52 | juxtapositional lobule |
| 239 | 0.050 | 3.38 | −40 | −18 | −34 | Left temporal fusiform cortex, posterior |
| Normal | ||||||
| 22394 | 0.000 | 4.78 | −28 | −54 | −8 | Left temporal occipital fusiform cortex |
| 3000 | 0.000 | 4.23 | −38 | −14 | 64 | Left precentral gyrus |
| 1539 | 0.000 | 4.58 | −2 | −10 | 48 | Juxtapositional lobule |
| 1486 | 0.000 | 4.25 | −26 | 6 | −6 | Left putamen |
| 1077 | 0.000 | 4.07 | 22 | 8 | −2 | Right putamen |
| 651 | 0.000 | 4.19 | −54 | 18 | 26 | Left inferior frontal gyrus, pars opercularis |
| 538 | 0.000 | 3.96 | 38 | 0 | 62 | Right middle frontal gyrus |
| Accelerated | ||||||
| 25090 | 0.000 | 4.69 | −40 | −52 | −20 | Left temporal occipital fusiform cortex |
| 2057 | 0.000 | 3.98 | −44 | −6 | 60 | Left precentral gyrus |
| 1882 | 0.000 | 4.28 | 38 | −2 | 62 | Right precentral gyrus |
| 1210 | 0.000 | 3.92 | 38 | 20 | −2 | Right insular cortex |
| Letter reading | ||||||
| Constrained | ||||||
| 51627 | 0.000 | 5.33 | −48 | −72 | −18 | Left lateral occipital cortex, inferior |
| Normal | ||||||
| 14762 | 0.000 | 4.69 | 0 | −4 | 44 | Cingulate gyrus, anterior |
| 12928 | 0.000 | 4.58 | 6 | −78 | −16 | Right lingual gyrus |
| 2320 | 0.000 | 4.27 | 28 | −2 | 0 | Right putamen |
| 2064 | 0.000 | 4.56 | −24 | 4 | 0 | Left putamen |
| 241 | 0.050 | 3.33 | −58 | 12 | 20 | Left inferior frontal gyrus, pars opercularis |
| Accelerated | ||||||
| 32271 | 0.000 | 4.5 | 8 | −78 | −18 | Right lingual gyrus |
| 341 | 0.010 | 3.32 | 38 | 40 | 24 | Right frontal pole |
| 314 | 0.020 | 3.69 | −34 | 42 | 22 | Left frontal pole |
Locations are cluster maximum Z values, labeled via Harvard‐Oxford Cortical Atlas.
In equivalent contrasts (constrained, normal, accelerated fluent sentence reading > rest; Fig. 2, left; Table I), fluent sentence reading activated regions including more medial occipito‐temporal regions than the letter reading task, notably within the fusiform gyri (FFG) bilaterally. The left middle temporal gyrus (MTG), particularly the posterior extent, and left IFG were also activated in contrasts at all speeds. At a constrained speed, an additional left MTG cluster and bilateral IFG activation were also observed. Anterior cingulate cortex was also more active bilaterally (left > right).
Sensitivity of Reading Regions' Activation During Fluent Sentence Reading
Whether the regions engaged during fluent sentence reading responded selectively or were also engaged in lower‐order processes was examined by directly comparing fluent sentence reading and letter reading. A direct contrast of these conditions (fluent sentence reading [all speeds] > letter reading [all speeds]) revealed clusters in the left FFG (temporal region), left IFG, and MTG (Fig. 3, Table II). Regions of the right lingual/fusiform, inferior frontal, and left superior frontal gyri were also significantly more active during fluent sentence reading.
Figure 3.
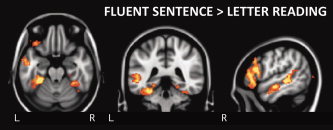
Fluent sentence reading > letter reading tasks (disregarding speed). Cluster thresholded (P<0.05, z>2.3), neurological space, saggital slice through left hemisphere (MNI coordinates −52, −40, −22). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Fluent sentence reading > letter reading disregarding reading speed
| Voxels | P | Z‐max | X | Y | Z | Location (Z‐max) |
|---|---|---|---|---|---|---|
| 3137 | 0.000 | 4.14 | 24 | −56 | −8 | Right lingual gyrus/occipital fusiform cortex |
| 2497 | 0.000 | 4.04 | −34 | −42 | −20 | Left temporal fusiform cortex, posterior |
| 1880 | 0.000 | 4.11 | −54 | 22 | 20 | Left inferior frontal gyrus, pars opercularis |
| 360 | 0.010 | 3.65 | −52 | −14 | −14 | Left middle temporal gyrus, anterior/posterior border |
| 326 | 0.010 | 4.19 | −52 | −32 | −4 | Left middle temporal gyrus, posterior |
| 320 | 0.010 | 3.62 | −6 | 14 | 58 | Left superior frontal gyus/SMA |
| 277 | 0.030 | 3.95 | 52 | 30 | 18 | right inferior frontal gyrus, pars triangularis |
Locations are cluster maximum Z‐values, labeled via Harvard‐Oxford Cortical Atlas.
A ROI analysis was then completed to examine the response within each region as reading speed varied (as described above; Fig. 4). The FFG region responded significantly more to fluent sentence reading than letter reading in both the constrained (t (24) = 3.629; P = 0.001) and accelerated (t (24) = 3.170; P = 0.004) conditions, but not so at a normal fluent reading speed (t (24) = 2.044; P = 0.052). The MTG responded preferentially to fluent sentence reading at constrained, normal, and accelerated speeds (t (24) = 5.076; P = 0.000; t (24) = 4.968; P = 0.000; t (24) = 4.341; P = 0.000, respectively) while the IFG, like the FFG, responded preferentially at constrained (t (24) = 4.868; P = 0.000) and accelerated (t (24) = 3.380; P = 0.002), but not normal (t (24) = 2.811; P = 0.010), reading speeds.
Effect of Altering Reading Speed
The effect of manipulating reading speed on reading‐related regions was initially examined by directly contrasting activation in constrained, normal, and accelerated speeded tasks for letter reading, and within the equivalent conditions for fluent sentence reading.
Generally, increasing letter reading speed above participants' normal reading rate led to activation changes in largely occipital and superior parietal regions (Fig. 5, right; Table III). Accelerated > constrained letter reading revealed clusters in the left lingual gyrus (a very posterior region bordering the occipital FFG, extending into bilateral cerebellum); bilateral parietal regions centered on the superior parietal lobule (left hemisphere), and supramarginal gyrus (right) extending medially to the posterior cingulate, and finally bilateral clusters through putamen, insula, and pallidus. Accelerated > normal letter reading revealed a similar picture. Clusters extended through parts of the parietal lobule (medially into the posterior cingulate) and into the supramarginal/angular gyri bilaterally, as well as the left lingual gyrus/cerebellum and the occipital pole. No regions were more active in normal as compared with constrained reading, and no regions decreased their activation as speed increased.
Figure 5.
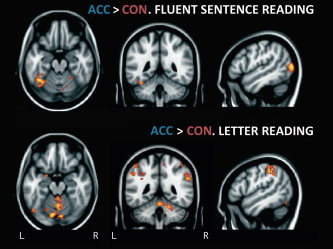
Accelerated (ACC) > constrained (CON) reading for fluent sentence (top) and letter (bottom) reading. Images cluster thresholded at P<0.05, z>2.3, neurological space, saggital slice through left hemisphere. MNI coordinates −50, −46, −20 (top) and −50, −46, −18 (bottom). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table III.
Between speed contrasts for fluent sentence reading
| Voxels | P | Z‐max | X | Y | Z | Location (Z‐max) |
|---|---|---|---|---|---|---|
| Increase in activation with speed | ||||||
| Accelerated > Constrained | ||||||
| 2520 | 0.000 | 3.69 | −38 | −54 | −20 | Left temporo‐occipital fusiform |
| 1903 | 0.000 | 3.96 | 48 | −78 | 8 | Right lateral occipital cortex (inferior) |
| Accelerated > Normal | ||||||
| 803 | 0.000 | 3.82 | −48 | −78 | 10 | Left lateral occipital cortex (inferior) |
| 636 | 0.000 | 3.82 | 48 | −76 | 8 | Right lateral occipital cortex (inferior) |
| 263 | 0.010 | 3.48 | 38 | 20 | −2 | Right insula |
| Normal > Constrained | ||||||
| 360 | 0.000 | 3.66 | −28 | −50 | −14 | Left temporo‐occipital fusiform |
| 308 | 0.000 | 3.02 | 22 | −46 | −18 | Right temporo‐occipital fusiform |
| Decrease in activation with speed | ||||||
| Constrained > Normal | ||||||
| 307 | 0.000 | 3.56 | 54 | 20 | 22 | Right inferior frontal gyrus |
Locations are cluster maximum Z values, labeled via Harvard‐Oxford Cortical Atlas.
Increasing fluent sentence reading speed was associated with greater activity in multiple regions, most notably the temporal fusiform region (Fig. 5, left; Table IV). When participants read at their normal rather than constrained fluent sentence reading speed, the maximal difference fell within the left temporal FFG, with a smaller cluster in a similar right hemispheric region. The contrast of accelerated > normal fluent sentence reading showed greater activation in lateral occipital cortex bilaterally, with the left hemisphere cluster extending into the inferior temporal gyrus and to a lesser extent the FFG, in a region abutting that revealed by the previous contrast. Activation was also greater within the right insula. Finally, a direct comparison of accelerated > constrained fluent sentence reading revealed greater activity in diffuse medial and lateral occipital regions and a separate, focal part of the left FFG.
Table IV.
Between speed contrasts for letter reading
| Voxels | P | Z‐max | X | Y | Z | Location (Z‐max) |
|---|---|---|---|---|---|---|
| Increase in activation with speed | ||||||
| Accelerated > Constrained | ||||||
| 2204 | 0.000 | 3.79 | −10 | −76 | −14 | Left lingual gyrus |
| 1993 | 0.000 | 3.58 | −32 | −58 | 56 | Left superior parietal lobule |
| 829 | 0.000 | 3.37 | 32 | 4 | −8 | Right cerebral white matter |
| 621 | 0.000 | 3.28 | −16 | 0 | 0 | Left pallidum |
| 386 | 0.000 | 3.23 | 56 | −44 | 32 | Right supramarginal gyrus, posterior |
| Accelerated > Normal | ||||||
| 1449 | 0.000 | 3.26 | −24 | −64 | 54 | Left lateral occipital cortex, superior |
| 806 | 0.000 | 3.25 | −10 | −76 | −16 | Left lingual gyrus |
| 530 | 0.000 | 3.34 | 56 | −44 | 34 | Right supramarginal gyrus, posterior |
| 312 | 0.010 | 3.1 | 6 | −92 | 16 | Right occipital pole |
Locations are cluster maximum Z values, labeled via Harvard‐Oxford Cortical Atlas.
As noted, when conditions of different durations are contrasted there is the possibility that significant differences in beta weights may be biased by differing model fit and may not reflect true differences in BOLD signal. We therefore also directly examined the BOLD response to stimuli at the point of peak difference in contrasts between conditions of different speeds. In all instances, the direction of the effect was as indicated in the contrasts. For fluent sentence reading, data for contrasts in which activation increased with speed indicated a standard canonical‐type response. That in which activation decreased (slow > normal) showed a drop in activation in the normal condition 2–4 s post‐stimulus onset (results for the key contrast, accelerated > constrained fluent sentence reading, are shown in Fig. 6). Data for the significant letter reading contrasts (accelerated > constrained, accelerated > normal) also revealed effects in the expected direction, with BOLD signal response shape differing somewhat from a canonical HRF.
Figure 6.
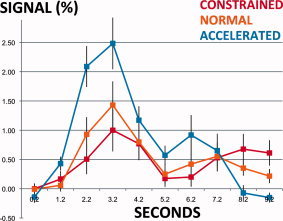
FFG BOLD signal response during constrained, normal, and accelerated fluent sentence reading. Scale: 0 and 1 are minimum and maximum BOLD responses for the constrained reading condition. Error bars: standard error of measurement. FFG region: point of peak difference for contrast [accelerated > constrained fluent sentence reading]. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
In contrast to a low‐level (rest) baseline, fluent sentence reading at normal, constrained, and accelerated speeds engaged a common set of regions typically activated in tasks assessing reading sub‐skills. These regions included occipito‐temporal cortex (notably bilateral fusiform regions), left MTG, left IFG, paracentral cortical regions as well as the anterior cingulate. Comparison with a higher‐level baseline task requiring basic orthographic processing more discretely revealed the expected regions typically implicated in reading [e.g., Pugh et al., 2001] including the left FFG, MTG, and IFG. ROI analyses showed that the left MTG was selectively more active for fluent sentence reading (than letter reading) regardless of reading speed, while the left FFG and IFG responded significantly more to fluent sentence reading at constrained and accelerated‐but not normal‐reading speeds, suggesting the reading system is well tuned and automatic at a normal fluent reading speed. The variation in the selectivity of the IFG and FFG's response during fluent sentence reading is in line with a multi‐componential view of reading fluency, in which the role played by all reading sub‐skills‐including comprehension‐is emphasized.
The direct comparison of fluent sentence reading at different speeds demonstrated that as speed increased key reading‐related regions responded differently. Engagement of the left MTG and IFG, implicated in the processing of phonological and semantic information, did not increase with speed. In contrast, activation of the FFG did. The fusiform region is frequently implicated in the processing of the visual characteristics of words as compared with other word‐like visual stimuli [e.g., Baker et al., 2007; Brem et al., 2006; Petersen et al., 1988]. For instance, this region has been shown to respond to English words and consonant strings more than numbers, line drawings, or Hebrew or Chinese characters in unilingual English speakers, and to become specialized through development [Baker et al., 2007; Brem et al., 2006]. Such findings have led to its being termed the “visual word form area,” though the specificity of this region's processing remains a topic of debate [e.g., Cohen et al., 2000; Price and Devlin, 2003]. In general, our data support the idea that this region's activation is directly and preferentially modulated by word stimuli as compared with non‐word consonant strings [e.g., Cohen et al., 2000].
The fact that reading‐related regions responded differently as the ability to read fluently was manipulated is also supportive of a multi‐componential view of reading fluency [Kame'enui and Simmons, 2001; Wolf and Katzir‐Cohen, 2001]. Here engagement of all reading‐related regions, including those more closely tied to comprehension [one of which is the IFG; Booth et al. 2002b], vary with fluency. We do not rule out a traditional view of reading fluency based on these results, however, as the IFG is not a discrete module supporting comprehension. The IFG can further be engaged by simply reading words aloud [see review in Turkeltaub et al., 2002] and temporal structures are engaged in comprehension and semantic processing [e.g., Price et al., 1997]. Regardless of theoretical orientation, these findings underscore the importance of looking beyond word‐level processes to reading sub‐skills such as orthographic, phonological, and semantic processing to gain a comprehensive understanding of fluent reading.
One implication of this view is that improvements in reading fluency might be achieved through targeting different lower‐order reading sub‐skills. For example, this study demonstrated that varying fluency through reading speed in adults increases BOLD signal in a brain region implicated in orthographic processing [e.g., Baker et al., 2007]. Targeting orthographic (in addition to phonological or semantic) processes might therefore improve connected‐text level reading fluency in readers who are fluent but read slowly. This focus may be particularly important for individuals who experience enduring fluency deficits in spite of phonological remediation. It also provides support for remediation methods that target multiple reading sub‐skills (e.g., orthography, phonology, semantics, syntax, morphology) as well as the relationship between these during development (i.e., the Reading Automaticity through Vocabulary, Engagement, and Orthography (RAVE‐O) program; Wolf et al. [2000b]). This approach can lead to improvements in multiple component reading skills which are in some instances greater than those seen when sub‐skills are targeted in isolation [Morris et al., 2010; see Wolf et al., 2009 for further discussion].
While caution is necessary when extrapolating our findings to the developing brain, a componential view of reading implies that reading fluency develops in concert with reading sub‐skills. Aspects of reading fluency might therefore be targeted at the earliest stages of reading development, and it will be important to further examine reading fluency loads on different sub‐skills through development. For instance, the relationship between fluency and phonological awareness is often being found to diminish with age while the relationship between fluency and rapid naming often remains significant [e.g., de Jong and van der Leij, 1999]. Indeed, in one recent study, the association between phonological awareness and reading fluency was found to decrease across reading development while the relationship between reading fluency and rapid automatized naming (RAN) increased [Vaessen and Blomert, 2010].
Specifically, this study demonstrates that it is important to look beyond word‐level processes to gain a comprehensive understanding of the neural structures supporting reading. Word‐level conceptualizations of reading [e.g., dual‐route and triangle models; Coltheart et al., 2001; Seidenberg and McClelland, 1989; see review in Coltheart, 2006] have provided critical and clinically useful insight into the structure of reading through modeling reading and reading disorder. Connected‐text level fluency is not simply explained by lower‐order sub‐skills, however; fluency deficits are a distinct element of dyslexia [e.g., Lyon et al., 2003; British Psychological Society, 1999] which may survive sub‐skill level remediation [Lyon and Moats, 1997]. Sub‐skill level abilities predict different aspects of reading fluency in distinct ways [Katzir et al., 2006] and deficits in different sub‐skills confer differing fluency deficits [Katzir et al., 2008]. While we have focused on fluent reading in typical adults, future studies of reading development and reading disordered populations will be necessary.
The cognitive structure of the RAN task (which involves the rapid naming of letters, numbers, colors, and objects) has also been a matter of considerable debate. Historically, many reading researchers have conceptualized RAN as largely reflecting phonological processes [see e.g., Wagner and Torgesen, 1987; detailed discussion in Wolf and Bowers, 1999]. More recently it has been seen as an index of multiple sub‐skills, especially orthography, and as engaging multiple brain regions [e.g., Misra et al., 2004]. To the extent that our letter reading task mirrors the RAN, our findings connect to this more multi‐component view of RAN, and to the contribution of more basic visual processes.
This study underscores the importance of considering the influence of reading speed on brain activation in studies of reading in general. As evidenced in our pre‐scanning assessment, even typical adult readers read at a range of speeds, and if text is presented at a uniform rate [as in e.g., Yarkoni et al., 2008], slower readers are essentially completing a more difficult task which will influence fusiform activation and study results. If between‐group differences in reading speed exist, this would constitute a systematic bias in study findings. For example, while individuals with and without reading disabilities might complete a task in which text is presented at a constant rate (say, 300 ms/word), such a rate may correspond to a “comfortable” reading rate for controls but a subjectively “fast” rate for the reading disabled group [e.g., Lovett, 1987; Martelli et al., 2009; O'Brien et al., 2005]. It is therefore interesting that studies of component reading skills in dyslexia and reading disability frequently report relative underactivation of the posterior reading network, including occipito‐temporal and temporal (e.g., MTG, inferior temporal gyrus) regions [e.g., see discussion in Shaywitz and Shaywitz, 2008]. Such findings therefore suggest a fundamental difference in neural functioning, as has been concluded in studies of reading skills in dyslexia that consider both task difficulty (reading level) and participant age [Hoeft et al., 2006]. The absence of an apparent increase in IFG activity with greater reading speed in this study is also notable in light of the frequent finding of IFG overactivation in dyslexia [Shaywitz and Shaywitz, 2008]. While our failure to observe a difference in activation is not proof that IFG activation does not increase with reading speed (discussed below), if this finding holds with further research it too would be in line with findings of primary reading‐related neural changes in dyslexia.
The observation of increased FFG activation with greater reading speed contrasts somewhat with the findings of Karni et al. [2005], which suggested less activation in accelerated as compared with constrained sentence reading in typical adults (though activation at different reading speeds was not directly statistically compared). They also observed significantly less activation in a right posterior temporal region during fast reading in reading impaired as compared with non‐impaired adults. This was considered in reference to the “acceleration phenomenon” described by Breznitz [e.g., see Breznitz, 1997; Breznitz and Leikin, 2001]. Here greater reading speed leads to improved decoding and comprehension; acceleration is thought to cause graphemic information to be processed differently than at a slower reading speed. This phenomenon is associated with earlier event‐related potential latencies for specific components in faster reading conditions [e.g., P200, P300 components; e.g., Breznitz and Leikin, 2001; see discussion in Karni et al., 2005]. The fusiform region is frequently implicated in the processing of the visual characteristics of words as compared with other word‐like visual stimuli [e.g., Baker et al., 2007; Brem et al., 2006; Petersen et al., 1988]. In concert with our findings, this model of the fusiform's role in reading suggests that when reading speed is accelerated and the ability to read fluently altered, regions engaged in reading respond selectively with lower‐order components engaged in word perception becoming more engaged.
It is possible that the greater FFG activity in the accelerated condition is driven by either increased rate of presentation or decreased event duration, as both these factors differed from the constrained condition. Some early studies of single word reading directly investigated the influence of inter‐stimulus interval (ISI) and event duration on activation. Price et al. [1994] found that reducing duration/increasing ISI led to a diffuse increase in left‐hemisphere activation during silent word reading, though inferior occipital regions were more active in a longer duration/shorter ISI condition. Leff et al. [2001] held stimulus duration constant and varied ISI; participants viewed single syllable words for 500 ms at rates of 5, 20, 40, 60, and 80 words per minute. Typical participants (n = 5) were reported to show a near linear increase in ventral prestriate engagement as ISI decreased. Together these findings suggest that the decreased ISI in our accelerated condition may be a key factor driving increased fusiform activation.
The complicating issue of BOLD signal nonlinearity needs to be considered in interpreting our findings. While observed differences between conditions of the same duration are valid (as model fit is equivalent), differences between conditions with different event durations may not be. Specifically, while analysis software scales the BOLD signal response to stimuli of different durations in a linear manner, the hemodynamic response is actually nonlinear [e.g., Boynton et al., 1996; Glover, 1999; for discussion see Birn and Bandettini, 2005]. In effect, when a contrast of beta weights for conditions of different durations reveals significant differences these may reflect (a) true differences in task‐related BOLD signal, and/or (b) biasing of the beta weights via differing model fit. To be certain our results reflected (a), we further examined the raw BOLD signal. These data supported the results of the between‐condition contrasts and our finding of an increase in left FFG engagement with greater reading speed. We cannot discount the possibility that our failure to observe speed‐related changes through other reading‐related regions (e.g., the left MTG, IFG) was a function of differences in model fit, however. The study's relatively small sample size may also have diminished our ability to detect changes in these and other regions.
We chose to equate task difficulty by determining participants' “comfortable” reading speed on an individual basis. This approach avoids the pitfalls of setting a task's reading speed at the group level, but it is necessary to rely on participants' individual reports of what reading speed is “comfortable” for them. Our experimental design also meant that all stimulus words (e.g., a six letter noun or a two letter function word) would be presented for an equivalent duration. Participants therefore likely read words for different amounts of time while each one was on screen (e.g., it may take someone a longer time to read the six letter noun than the two letter function word). The use of a self‐paced reading paradigm may arguably have allowed participants to read at a “comfortable” speed more effectively, though manipulation of reading speed is difficult in such a paradigm. At the stimulus level, we did not account for orthographic neighborhood effects. We note, however, that while lexicality × orthographic neighborhood effects have been observed in medial PFC and mid‐dorsolateral regions [Fiebach et al., 2007], we did not observe key effects in these regions. A conceptual issue relates to our decision to vary reading fluency through reading speed while ensuring comprehension. While this does alter a core aspect of reading fluency [rapid, accurate reading with comprehension; e.g., Lyon et al., 2003; NRP, 2000] it will be important to compare our findings with those obtained when accuracy or comprehension are manipulated, or when larger passages of text are used. This may be harder to implement [e.g., using a natural stimulation type of design as described in Hasson et al. 2010] but could also address other issues such as low statistical power. It is necessary for assessments of fluent reading to demonstrate ceiling level performance, as fluency entails accurate reading with comprehension. With comprehension and accuracy constrained, the neural changes accompanying variation in reading speed are inherently of interest, as they reflect real changes in the cognitive/neural structures supporting real‐word, naturalistic reading. An interesting alternate approach to studying fluency, however, would manipulate fluency through task accuracy. Additionally, future studies could relate neural function to standard behavioral measures.
Clear extensions of this study include examining changes in the neural correlates of fluent reading in development through childhood and in reading disability. There is an increasing recognition that rather than being a disorder caused by a single core deficit, dyslexia can arise from multiple causes. Accordingly, key deficits have been reported in dyslexia in phonological processing and rapid naming/fluency processes [Katzir et al., 2008; Wolf et al., 2000a]. Given the multi‐componential nature of reading fluency and the pervasiveness of fluency deficits [e.g., Lyon et al., 2003], it is likely that such deficits will relate to differing neural profiles. This may assist in identification of subtypes of dysfluency. Our task also provides the opportunity to examine fluency development longitudinally, and (with behavioral measures) identify early behavioral markers of later dysfluency.
CONCLUSIONS
Fluent sentence reading was associated with activation through regions known to be engaged by reading sub‐skills, including the bilateral FFG, MTG, and IFG. Comparison with an appropriate control task indicated that the left MTG consistently responded preferentially to fluent sentence reading as compared with the processing of matched consonant strings. Bilateral FFG and IFG responded selectively more during accelerated and constrained, but not normal, reading speeds. Direct contrasts between the reading tasks performed at different speeds indicated that in higher‐order, naturalistic sentence‐level fluent reading the fusiform gyrus both (a) responds to meaningful word stimuli and (b) increases its activity when fluent reading speed is accelerated. These findings are in line with a view that understands fluency as the product of multiple contributing reading sub‐skills. They underscore the importance of understanding the relationships between the development of multiple reading sub‐skills and fluency across development, and that it is necessary to look beyond lower‐level reading processes (e.g., phonological processing) to gain a comprehensive understanding of how the brain reads. Finally, a clear profile of the relationship between reading sub‐skills and fluency at a neural level can inform future studies of fluent reading and raises the possibility of subtyping differing forms of dysfluency through behavioral and neural measures.
Acknowledgements
Authors thank Professor Maryanne Wolf for her generous assistance with drafts of this document, and Michelle Lee, Rachel Steinhorn and Cora Smayda for assistance with data collection, analysis and stimulus preparation. Authors also thank two anonymous reviewers for feedback which significantly improved this document.
REFERENCES
- Baker CI, Liu J, Wald LL, Kwong KK, Benner T, Kanwisher N ( 2007): Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc Natl Acad Sci USA 104: 9087–9092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM ( 2003): General multilevel linear modeling for group analysis in fMRI. Neuroimage 20: 1052–1063. [DOI] [PubMed] [Google Scholar]
- Berninger VW, Abbott RD, Billingsley F, Nagy W ( 2001): Processes underlying timing and fluency of reading. Dyslexia, fluency, and the brain 383–414. [Google Scholar]
- Birn RM, Bandettini PA ( 2005): The effect of stimulus duty cycle and. Neuroimage 27: 70–82. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM ( 2002a): Modality independence of word comprehension. Hum Brain Mapp 16: 251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM ( 2002b): Functional anatomy of intra‐ and cross‐modal lexical tasks. Neuroimage 16: 7–22. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ ( 1996): Linear systems analysis of functional magnetic resonance imaging in human v1. J Neurosci 16: 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem S, Bucher K, Halder P, Summers P, Dietrich T, Martin E, Brandeis D ( 2006): Evidence for developmental changes in the visual word processing network beyond adolescence. Neuroimage 29: 822–837. [DOI] [PubMed] [Google Scholar]
- Breznitz Z ( 1997): Reading rate acceleration: Developmental aspects. J Genet Psychol 158: 427–441. [DOI] [PubMed] [Google Scholar]
- Breznitz Z, Leikin M ( 2001): Effects of accelerated reading rate on processing words' syntactic functions by normal and dyslexic readers: Event related potentials evidence. J Genet Psychol 162: 276–296. [DOI] [PubMed] [Google Scholar]
- British Psychological Society ( 1999): Dyslexia, Literacy, and Phonological Assessment. Leicester, UK: British Psychological Society. [Google Scholar]
- Burns P, Roe B ( 2001): Informal Reading Inventory: Preprimer to Twelfth Grade. Boston: Houghton Mifflin. [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene‐Lambertz G, Henaff MA, Michel F. ( 2000): The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split‐brain patients. Brain 123: 291–307. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J ( 2001): DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol Rev 108: 204–256. [DOI] [PubMed] [Google Scholar]
- Coltheart M ( 2006): Dual route and connectionist models of reading: An overview. London Rev Educ 4: 5–17. [Google Scholar]
- Cooke A, Grossman M, DeVita C, Gonzalez‐Atavales J, Moore P, Chen W, Gee J, Detre J ( 2006): Large‐scale neural network for sentence processing. Brain Lang 96: 14–36. [DOI] [PubMed] [Google Scholar]
- de Jong PF, van der Leij A ( 1999): Specific contributions of phonological abilities to early reading acquisition: Results from a Dutch latent variable longitudinal study. J Educ Psychol 91: 450. [Google Scholar]
- Fiebach CJ, Ricker B, Friederici AD, Jacobs AM ( 2007): Inhibition and facilitation in visual word recognition: Prefrontal contribution to the orthographic neighborhood size effect. Neuroimage 36: 901–911. [DOI] [PubMed] [Google Scholar]
- Glover GH ( 1999): Deconvolution of impulse response in event‐related bold fMRI. Neuroimage 9: 416–429. [DOI] [PubMed] [Google Scholar]
- Hasbrouck JE, Tindal G ( 1992): Curriculum‐based oral reading fluency norms for students in grades 2 through 5. Teach Exceptional Child 24: 41–44. [Google Scholar]
- Hasson U, Malach R, Heeger DJ ( 2010). Reliability of cortical activity during natural stimulation. Trends Cogn Sci 14: 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF ( 1999): Semantic cortical activation in dyslexic readers. J Cogn Neurosci 11: 535–550. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor‐Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA ( 2006): Neural basis of dyslexia: A comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci 26: 10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Vigneau M, Mazoyer B, Tzourio‐Mazoyer N ( 2007): Impact of modality and linguistic complexity during reading and listening tasks. Neuroimage 34: 784–800. [DOI] [PubMed] [Google Scholar]
- Kame'enui EJ, Simmons DC ( 2001): Introduction to this special issue: The DNA of reading fluency. Scientific Studies Read 5: 203–210. [Google Scholar]
- Kame'enui E, Simmons D, Good R, Harn B. Processes underlying timing and fluency of reading: Efficiency, automaticity, coordination, and morphological awareness In Wolf M, editor. 2001. Dyslexia, Fluency, and the brain. Maryland: York Press; p 383–314. [Google Scholar]
- Karni A, Morocz IA, Bitan T, Shaul S, Kushnir T, Breznitz Z ( 2005): An fMRI study of the differential effects of word presentation rates (reading acceleration) on dyslexic readers' brain activity patterns. J Neurolinguist 18: 197–219. [Google Scholar]
- Katzir T, Kim Y, Wolf M, O'Brien B, Kennedy B, Lovett M, et al. ( 2006): Reading fluency: The whole is more than the parts. Ann Dyslexia 56: 51–82. [DOI] [PubMed] [Google Scholar]
- Katzir T, Kim Y, Wolf M, O'Brien B, Kennedy B, Lovett M, Morris R ( 2008): The varieties of pathways to dysfluent reading: Comparing subtypes of children with dyslexia at letter, word, and connected text levels of reading. J Learn Disabil 41: 47. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H ( 2006): Evidence for a dysfunction of left posterior reading areas in german dyslexic readers. Neuropsychologia 44: 1822–1832. [DOI] [PubMed] [Google Scholar]
- Kujala J, Pammer K, Cornelissen P, Roebroeck A, Formisano E, Salmelin R ( 2007): Phase coupling in a cerebro‐cerebellar network at 8–13 Hz during reading. Cereb Cortex 17: 1476–1485. [DOI] [PubMed] [Google Scholar]
- Leff AP, Crewes H, Plant GT, Scott SK, Kennard C, Wise RJS ( 2001): The functional anatomy of single‐word reading in patients with hemianopic and pure alexia. Brain 124:510. [DOI] [PubMed] [Google Scholar]
- Lovett MW ( 1987): A developmental approach to reading disability: Accuracy and speed criteria of normal and deficient reading skill. Child Dev 58: 234–260. [PubMed] [Google Scholar]
- Lyon G, Moats L ( 1997): Critical conceptual and methodological considerations in reading intervention research. J Learn Disabil 30: 578. [DOI] [PubMed] [Google Scholar]
- Lyon GR, Shaywitz SE, Shaywitz BA ( 2003): Defining dyslexia, comorbidity, teacher's knowledge of language and reading. Ann Dyslexia 53: 1–14. [Google Scholar]
- Martelli M, Di Filippo G, Spinelli D, Zoccolotti P ( 2009): Crowding, reading, and developmental dyslexia. J Vis 9: 14.1–14.18. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield‐Gabrieli S, Gabrieli JD, Just MA ( 2007): Brain activation during sentence comprehension among good and poor readers. Cereb Cortex 17: 2780–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Katzir T, Wolf M, Poldrack R ( 2004): Neural systems for rapid automatized naming in skilled readers: Unraveling the ran‐reading relationship. Scientific Studies Read 8: 241. [Google Scholar]
- Morris RD, Lovett MW, Wolf M, Sevcik RA, Steinbach KA, Frijters JC, Shapiro MB (In press): Multiple‐component remediation for developmental reading disabilities: IQ, socioeconomic status, and race as factors in remedial outcome. J Learn Disabil. National Reading Panel ( 2000). Chapter 3: Fluency. In Report of the National Reading Panel, retrieved from http://www. nationalreadingpanel.org/publications/publications.htm May 5, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien BA, Mansfield JS, Legge GE ( 2005): The effect of print size on reading speed in dyslexia. J Res Read 28: 332–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME ( 1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331: 585–589. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT ( 2003): The myth of the visual word form area. Neuroimage 19: 473–481. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Watson JDG, Patterson K, Howard D, Frackowiak RSJ ( 1994): Brain activity during reading: The effects of exposure duration and task. Brain 117:1255. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS ( 1997): Segregating semantic from phonological processes during reading. J Cogn Neurosci 9: 727–733. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA ( 2000): Functional neuroimaging studies of reading and reading disability (developmental dyslexia). Ment Retard Dev Disabil Res Rev 6: 207–213. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA ( 2001): Neurobiological studies of reading and reading disability. J Commun Disord 34: 479–492. [DOI] [PubMed] [Google Scholar]
- Rimrodt SL, Clements‐Stephens AM, Pugh KR, Courtney SM, Gaur P, Pekar JJ, Cutting LE ( 2009): Functional MRI of sentence comprehension in children with dyslexia: Beyond word recognition. Cereb Cortex 19: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels S ( 2006): Toward a model of reading fluency In: Samuels S, Farstrup A, editors. What Research has to Say About Fluency Instruction. Newark, USA: International Reading Association; pp 4–23. [Google Scholar]
- Schlaggar BL, McCandliss BD ( 2007): Development of neural systems for reading. Annu Rev Neurosci 30: 475–503. [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, van der Mark S, Bucher K, Brem S, Martin E, et al. ( 2009): Reading for meaning in dyslexic and young children: Distinct neural pathways but common endpoints. Neuropsychologia 47: 2544–2557. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL ( 1989): A distributed, developmental model of word recognition and naming. Psychol Rev 96: 523–568. [DOI] [PubMed] [Google Scholar]
- Seymour PH ( 1987): Individual cognitive analysis of competent and impaired reading. Br J Psychol (London, England: 1953) 78: 483. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA ( 2008): Paying attention to reading: The neurobiology of reading and dyslexia. Dev Psychopathol 20: 1329–1349. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Lyon GR, Shaywitz SE ( 2006): The role of functional magnetic resonance imaging in understanding reading and dyslexia. Dev Neuropsychol 30: 613–632. [DOI] [PubMed] [Google Scholar]
- Shinn MR, Good R, Knutson N, Tilly W, Collins V ( 1992): Curriculum based measurement of oral reading fluency: A confirmatory analysis of its relation to reading. School Psychol Rev 21: 459–479. [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Mouzaki A, Papanicolaou AC ( 2001): Age‐related changes in regional brain activation during phonological decoding and printed word recognition. Dev Neuropsychol 19: 191–210. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 ( Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Temple E ( 2002): Brain mechanisms in normal and dyslexic readers. Curr Opin Neurobiol 12: 178–183. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Rashotte CA, Alexander AW ( 2001): Dyslexia, fluency, and the brain. In Wolf M, editor. Maryland: York Press; p 307–332. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA ( 2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16: 765–780. [DOI] [PubMed] [Google Scholar]
- Vaessen A, Blomert L ( 2010): Long‐term cognitive dynamics of fluent reading development. J Exp Child Psychol 105: 213–231. [DOI] [PubMed] [Google Scholar]
- van Atteveldt N, Formisano E, Goebel R, Blomert L ( 2004): Integration of letters and speech sounds in the human brain. Neuron 43: 271–282. [DOI] [PubMed] [Google Scholar]
- Wagner R, Torgesen JK ( 1987): The nature of phonological processing and its causal role in acquisition of reading skills. Psychol Bull 101: 192–212. [Google Scholar]
- Wolf M, Bowers PG ( 1999): The double‐deficit hypothesis for the developmental dyslexias. J Educ Psychol 91: 415–438. [Google Scholar]
- Wolf M, Katzir‐Cohen T ( 2001): Reading fluency and its intervention. Scientific Studies of Reading 5: 211–239. [Google Scholar]
- Wolf M, Bowers PG, Biddle K ( 2000a): Naming‐speed processes, timing, and reading: A conceptual review. J Learn Disabil 33: 387. [DOI] [PubMed] [Google Scholar]
- Wolf M, Miller L, Donnelly K ( 2000b): Retrieval, automaticity, vocabulary elaboration, orthography (rave‐o). J Learn Disabil 33: 375. [DOI] [PubMed] [Google Scholar]
- Wolf M, Barzillai M, Gottwald S, Miller L, Spencer K, Norton E, Lovett M, Morris R ( 2009): The RAVE‐O intervention: Connecting neuroscience to the classroom. Mind Brain Educ 3: 84–93. [Google Scholar]
- Yarkoni T, Speer NK, Balota DA, McAvoy MP, Zacks JM ( 2008): Pictures of a thousand words: Investigating the neural mechanisms of reading with extremely rapid event‐related fMRI. Neuroimage 42: 973–987. [DOI] [PMC free article] [PubMed] [Google Scholar]


