Figure 4. Structural causes of minimal specificity.
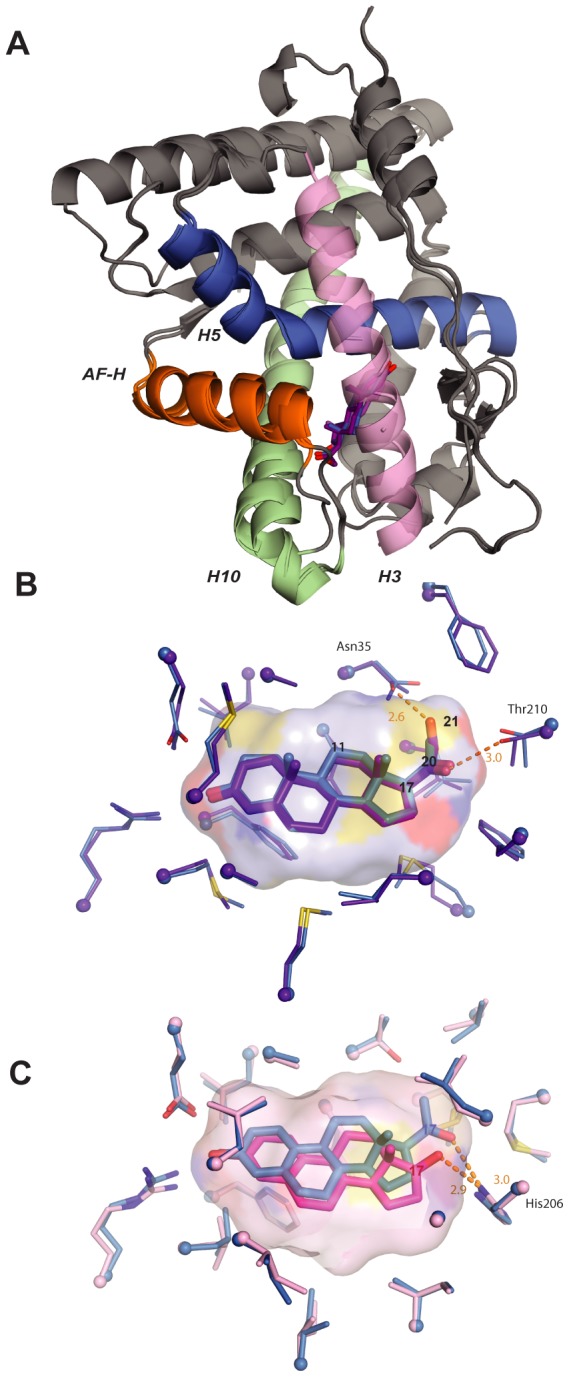
A, X-ray crystal structures of AncSR2 with progesterone (blue) and DOC (purple) are superimposed. Ligands are shown as sticks. Helices making major ligand contacts and the activation-function helix (AF-H) are shown in contrasting colors. B, Structural causes of promiscuity in AncSR2. The ligand cavity of the AncSR2-progesterone structure, shown as a surface, has adequate volume to accommodate the 21-hydroxyl of DOC. Ligand contacts in the crystal structures of AncSR2 with progesterone (blue) and DOC (purple) are shown. Thick sticks, ligand; thin sticks, side chains that contact ligand; balls, α-carbons. Steroid carbons 11, 17, 20, and 21 are numbered. Hydrogen bonds are shown as orange dotted lines. C, Structural basis for promiscuity in AncSR1. Ligand contacts in the AncSR1 model with estradiol (magenta) and NPT (blue) are shown. The cavity of the AncSR1-estradiol complex, which has adequate room to accommodate the 17-acetyl of NPT, is shown. Two side chains between the viewer and the ligand are hidden for clarity.
