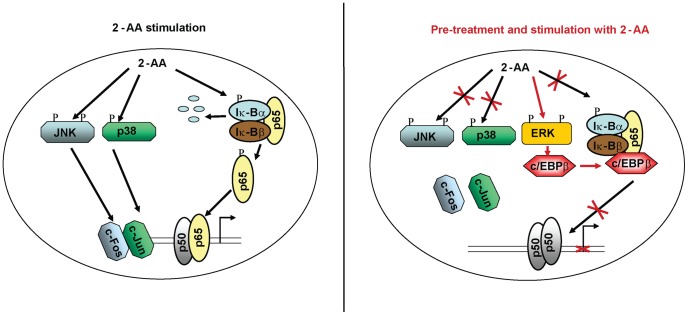
Figure 9. Proposed model for 2-AA immunomodulatory mechanisms.
In naïve cells (left), stimulation with 2-AA induces activation of NF-κB, which leads to the phosphorylation and degradation of I-κBα, releasing the NF-κB dimers p65 and p50. 2-AA also induces the p38 MAPK and JNK pathways to stimulate c-Jun and c-Fos. Activation of MAPK and NF-κB pathway upregulates pro-inflammatory genes. In contrast, in 2-AA pretreated cells (right) over-expression of ERK1/2 activates C/EBPβ, which binds directly to p65, resulting in c/EBPβ-p65 complex formation, and preventing 2-AA induced phosphorylation of p65 upon 2-AA stimulation. This interaction inhibits NF-κB mediated transactivation. The activation of JNK and p38 MAPK are repressed in 2-AA pretreated cells. All together, repression of the p38 MAPK, JNK, and NF-κB pathways abrogates the activation of pro-inflammatory mediators.