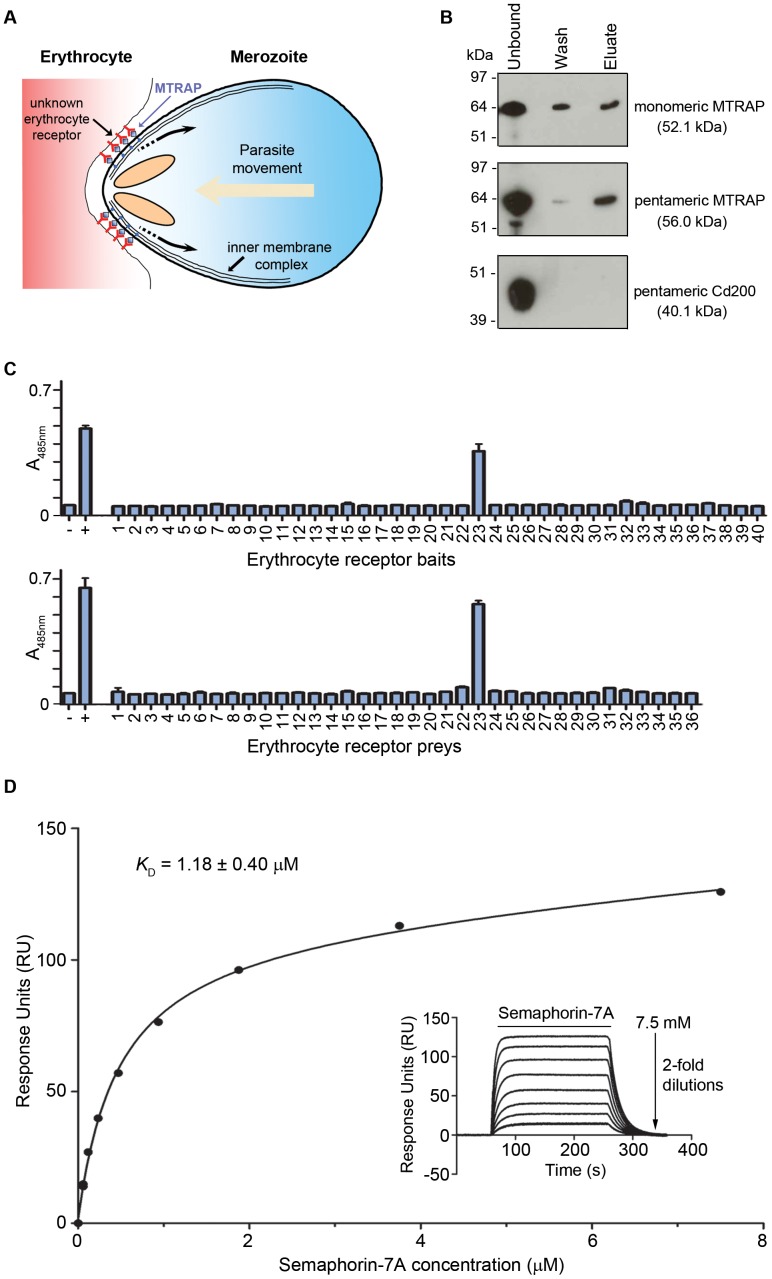
Figure 1. Semaphorin-7A is an erythrocyte receptor for MTRAP.
(A) Schematic diagram showing how TRAP-like ligands are thought to play a key bridging role between the parasite and the target cell that is to be invaded. In this case, the cytoplasmic region of MTRAP interacts with the parasite actin-myosin motor that provides the power necessary for invasion. The extracellular region of MTRAP interacts with an erythrocyte receptor thereby providing the necessary traction for forwards movement of the parasite, driving host cell invasion. (B) Purified monomeric and pentameric MTRAP bound human erythrocytes relative to a negative control (pentameric Cd200). Unbound, wash and eluted material was resolved under reducing conditions by SDS-PAGE and detected by Western blotting using an anti-His antibody. Predicted monomer molecular weights are indicated in brackets. The pentamers are expected to split into the constituent monomers upon reduction. (C) Systematic screening identifies Semaphorin-7A as an MTRAP receptor. MTRAP was screened against an erythrocyte receptor protein library using the AVEXIS assay, either as a prey against 40 erythrocyte baits (top panel) or as a bait against 36 erythrocyte preys (bottom panel). A single interaction with Semaphorin-7A (protein number 23) was identified in both bait–prey orientations. Bar graphs represent means ± SD, n = 3. (D) MTRAP and Semaphorin-7A directly interact. Serial dilutions of purified monomeric Semaphorin-7A were injected over MTRAP immobilised on a streptavidin-coated sensor chip until equilibrium had been achieved (inset). Reference-subtracted binding data were plotted as a binding curve and the equilibrium dissociation constant was calculated using non-linear regression fitting of a simple Langmuir binding isotherm to the data. A K D of 1.18±0.40 µM (mean ± SEM) was calculated from three independent experiments (Table S1). A representative experiment is shown.