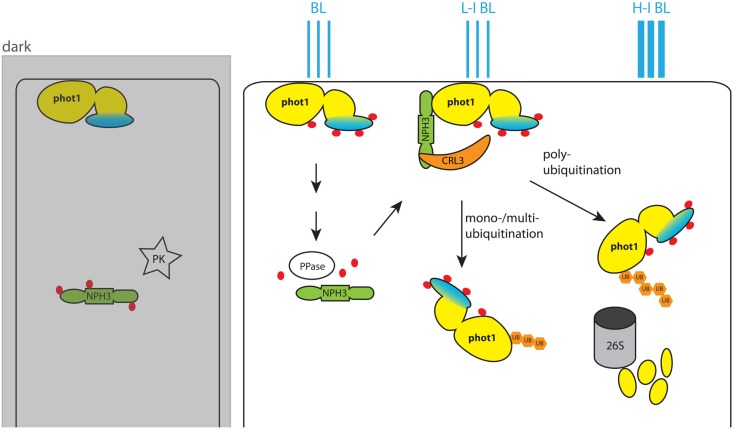
Figure 2.
Model for blue light (BL)-dependent regulation of phot1 activity. In the dark phot1 is unphosphorylated and associates to the PM. NPH3 on the other hand is phosphorylated by an unknown protein kinase (PK) and remains in the cytosol. BL triggers autophosphorylation of phot1, and this leads to dephosphorylation of NPH3 by an unidentified phot1-controlled protein phosphatase. Dephosphorylated NPH3 heterodimerizes with CUL3 and forms the CRL3NPH3 E3 ubiquitin ligase. In this ligase NPH3 functions as anchor for the PHOT1 substrate. In low intensity BL (L-I BL) the activity of CRL3NPH3 leads to mono-/multiubiquitination of PHOT1 and its subsequent localization to the cytoplasm. With higher BL intensities (H-I BL) CRL3NPH3 mediates polyubiquitination of phot1. This marks phot1 for degradation by the 26S proteasome and eventually desensitizes the plant cell for BL by reducing the amount of available receptors. The phot1 kinase domain is colored yellow, and the photoreceptor domain is marked in blue. Phosphate groups on the proteins are indicated by red dots, while ubiquitin groups are represented by orange hexagons.