Abstract
Background: Multiagent chemotherapy (MCT) has mitochondrial targets. Since technetium-99m-sestamibi (MIBI) is a marker of mitochondrial metabolism, cardiac MIBI uptake and MIBI washout rate (%WR) may detect MCT-induced cardiotoxicity. Methods: In 16 cancer patients on MCT for 10 months and in 14 non-cancer controls, cardiac MIBI uptake between early (30 min) and delayed (3 hours) post-injection planar images was measured as counts per pixel (cpp). The MIBI cardiac %WR was also measured. Results: When MCT patients and controls were compared, early and cardiac delayed MIBI uptake were greater in MCT patients (45 ± 12 cpp vs. 30 ± 4 cpp; p <0.04) and (30 ± 8 cpp vs. 25 ± 2 cpp; p < 0.02), but % WR did not change (12 ± 4% vs. 13 ± 3%; p = ns). However, in the MCT patients, the MIBI cardiac %WR was more rapid because it was obtained at the same time as in the control patients but from a greater amount of MIBI cardiac uptake. On 36-months follow-up, only MCT patients died of cardiac death. Overall survival risk parameters, only delayed cardiac MIBI uptake (Odds ratio = 1.7, p<0.001) and early cardiac MIBI uptake (Odds ratio = 1.2, p<0.02) were found to be significantly associated with cardiac mortality. Conclusions: In experimental studies, anticancer drugs elicit mitochondrial membrane hyperpolarization with passive cardiac MIBI uptake. In MCT patients, the increased cardiac MIBI uptake and rapid %WR compared with controls may reflect mitochondrial membrane dysfunction, pre-clinical cardiotoxicity and thus poor prognosis.
Keywords: Multiagent chemotherapy (MCT), technetium-99m-sestamibi (MIBI), mitochondrial metabolism, cardiotoxicity, cancer, multiagent chemotherapy
Introduction
Cancer therapy has progressed considerably, improving survival for many patients [1]. However, deaths related to the cardiotoxicity of anticancer drugs occur through mechanisms that are still unclear. Multiagent chemotherapy (MCT) and radiotherapy are cardiotoxic interventions that can lead to diverse complications, such as heart failure, myocardial ischemia, myocardial infarction, hypertension, thromboembolism, QT prolongation, hypotension and arrhythmias [2]. Despite such different clinical manifestations of cardiotoxicity, anticancer drugs may produce these effects through common cardiac mitochondrial targets [3]. Because technetium-99m-sestamibi (MIBI) is a marker of cardiac mitochondrial metabolism [4], cardiac MIBI uptake and washout on planar imaging, in addition to perfusion and left ventricular (LV) function on MIBI cardiac stress/rest gated-SPECT imaging, can detect pre-clinical signs of cardiotoxicity. Previous experimental studies have found that abnormal MIBI cardiac kinetics may reflect cardiac mitochondrial dysfunction [5]. There is, however, a lack of information regarding the effects of cardiac MIBI uptake and release in patients on MCT. This study evaluates the hypothesis that an abnormal cardiac MIBI kinesis could represent a pre-clinical sign of cardiotoxicity in MCT patients. These results could encourage physicians to administer an early and rational course of cardioprotection despite the presence of a normal ejection fraction. Sixteen cancer patients on MCT and fourteen non-cancer patients were therefore evaluated in a retrospective case-control study.
Materials and methods
Patient population
Sixteen consecutive cancer patients with a median age of 59 years (range 52-69; 9/16 (56%) males) were referred for a MIBI stress/rest gated-SPECT assessment due to complaints of functional capacity despite a normal ejection fraction after a median period of 10 months (range 8-21) on MCT.
The results were compared to those of 14 noncancer control patients with a median age of 58 years (range 57-69; 6/14 (43%) males) with similar symptoms who were also referred for MIBI cardiac stress/rest gated-SPECT assessment due to complaints of functional capacity despite a normal ejection fraction. The cancer and control patients were selected consecutively from Januay to July 2008 and did not differ with respect to age, gender or established cardiovascular risk factors, such as hypertension, dyslipidemia, diabetes and obesity (p > 0.05 for all). Atrial fibrillation or an ejection fraction < 45% were the exclusion criteria. The protocols for MCT were in adherence with the American Society of Clinical Oncology and the Oncology Nursing Society standards for safe chemotherapy administration [6]. All the patients provided written informed consent, and the local ethics committee approved the protocol of this study.
Imaging methods
Early anterior view planar imaging of the chest and stress-SPECT imaging were performed 30 min after stress injection of an average dose of 370 MBq of MIBI, commercially available as Cardiolite [7]. Delayed planar imaging was acquired 3 hours after the stress injection. A second injection of an average dose of 925 MBq of MIBI was made after delayed planar imaging and was followed after 1 hour by rest gated-SPECT imaging.The isotope dosage was tailored by considering the patients body mass index and measure of abdominal circunmference. Imaging was performed with a single-day protocol using a double-headed gamma camera (DST-XL; Sopha Medical Vision International, Buc, France) equipped with a low-energy, parallelbole, high-resolution collimator. On the planar images, early and delayed MIBI uptake were calculated as counts per pixel (cpp) in a region of interest (ROI), delineated by the contours of the heart area, with a ROI drawn in an area around the cannula used for injection and a ROI on the liver area (Figure 1). The MIBI washout rate (WR) was calculated in these 3 ROIs as previously defined [8]: % WR = early MIBI uptake – delayed MIBI uptake x Hf x 100/early MIBI uptake, where Hf = (1/(1/2)x and x = (T delayed imaging – T early imaging )/6; the T was defined as the time for delayed planar imaging and the time for early planar imaging. MIBI tomographic images were obtained by use of a 180-degree circular orbit, from 45-degree right anterior oblique to 45-degree left posterior oblique, 32-frame step-and-shoot, 60 sec/frame, with the patient in the supine position. Only rest projections were ECG-gated and 16 individual ECG-gated frames per cardiac cycle were acquired. The gate tolerance was 100%. All patients in this study had a sinus rhythm. To avoid misinterpretation of attenuation artifacts, images were evaluated by 2 independent observers blinded to the patients clinical data.
Figure 1.
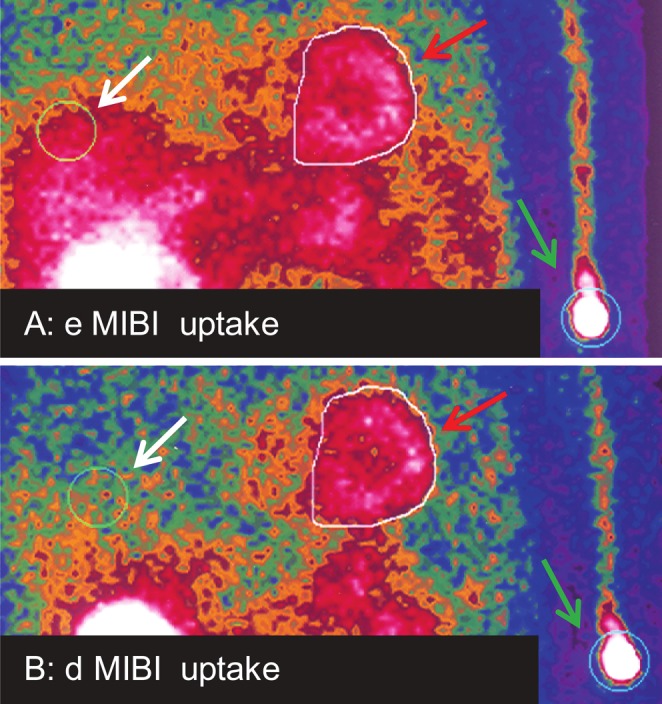
A. early (e) planar imaging 30 min after MIBI injection. B. delayed (d) planar imaging 3 hours after MIBI injection. ROIs for MIBI uptake calculation are shown: cardiac (red arrows), site of injection (green arrows), and liver (white arrows).
The symptom-limited exercise testing used for SPECT imaging was performed in 25 patients. Dipyridamole infusion was used in five patients who were unable to perform the exercise testing; 3/16 (19%) MCT patients with bone metastases were unable to perform the exercise testing, and 2/14 (12%) control patients were incapable of pedaling an exercise machine. Fixed and reversible abnormal perfusion defects were analyzed using a Cedars-Sinai protocol [9] with 1 denoting the presence of defects and 0 denoting an absence of defects (Figure 2). A 36-month clinical follow-up was performed.
Figure 2.
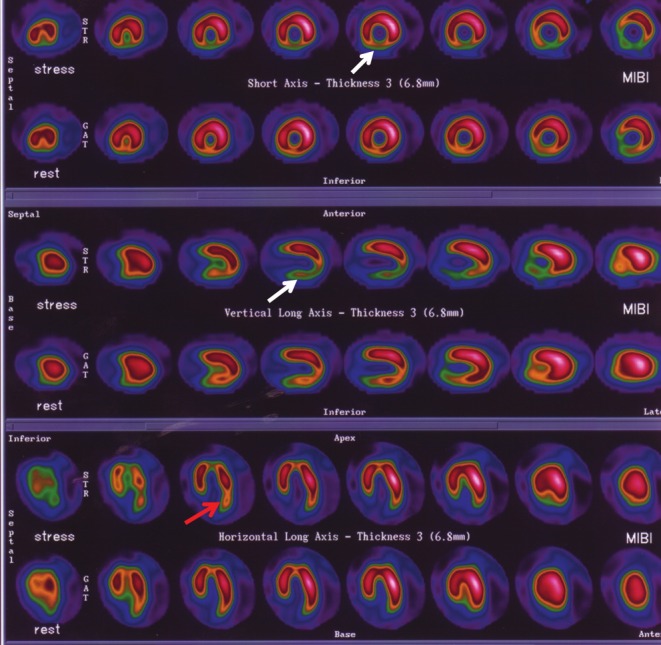
MIBI SPECT of a patient after MCT. An inferior reversible defect (white arrows) and a lateral reversible defect (red arrow) are shown. A coronary angiogram revealed an absence of coronary obstructions.
Statistical analyses
Statistical analyses were performed using MedCalc for Windows, version 12.0.3.0 (MedCalc Software, Broekstraat 52, 9030 Mariakerke, Belgium). The patient ages and the follow-up duration were expressed as median +/- interquartile ranges. All values were then expressed as mean ± standard deviation. The differences between means were evaluated by unpaired or paired t-tests. In cases of unequal variances, the t-test was corrected with the Welch test. The differences between percentages were calculated with Fisher's exact test. The effect of the risk parameters on survival was analyzed with a logistic regression. A receiver operating characteristic (ROC) curve was used to calculate the criterion value of the risk parameters on survival. In two groups of patients, survival curves were compared using the Kaplan-Meier analysis with the log-rank test. A p-value less than 0.05 was considered statistically significant.
Results
Of the MCT patients, 9/16 (56%) experienced early cardiovascular adverse events within one month of MCT in the period before planar and SPECT imaging. Specifically, 1 patient had myocardial ischemia, 3 had acute myocardial infarction, 2 had atrial fibrillation, 1 had atrial fibrillation and heart failure, 1 had supraventricular tachycardia, and 1 had a pulmonary embolism. On coronary angiograms, there was a lack of coronary obstruction in the 3 patients with myocardial infarction, but the patient with myocardial ischemia had single-vessel coronary artery disease. In the gated-SPECT imaging, all the patients showed normal ejection fractions, but abnormal perfusion defects were observed in only some MCT patients. Specifically, there were fixed perfusion defects in 2/16 (12%) MCT patients and reversible perfusion defects in 7/16 (44%) MCT patients. When MCT patients and controls were compared, early MIBI and delayed cardiac MIBI uptake were significantly greater in the MCT patients, but the cardiac MIBI % WR did not change. However, in the MCT patients, the MIBI cardiac % WR was more rapid because it was obtained at the same time as in the control patients but from a greater amount of MIBI cardiac uptake. When early and delayed MIBI uptake and MIBI % WR of the liver and injection area from the MCT patients were compared with the respective values in the controls, the results did not differ. The cardiac MIBI kinetics profile of the patients are summarized in Table 1.
Table 1.
The cardiac MIBI kinetics profile of the patients
| MCT patients n=16 | p | Controls n=14 | |
|---|---|---|---|
| Early cardiac MIBI uptake (ccp) | 45±12 | 0.04 | 30±4 |
| Delayed cardiac MIBI uptake (ccp) | 30±8 | 0.02 | 25±2 |
| Cardiac % WR | 12±4 | ns | 13±3 |
| Early liver MIBI uptake (ccp) | 56±22 | ns | 50±15 |
| Delayed liver MIBI uptake (ccp) | 23±9 | ns | 21±10 |
| Liver % WR | 36±10 | ns | 37±8 |
| Early MIBI uptake (ccp) of the area of MIBI injection | 189±185 | ns | 145±41 |
| Delayed MIBI uptake (ccp) of the area of MIBI injection | 147±138 | ns | 113±34 |
| MIBI % WR of the area of MIBI injection | 21±4 | ns | 22±5 |
| Patients body mass index (kg/m2) | 27±6 | ns | 28±4 |
| Patients abdominal circumference (cm) | 100±15 | ns | 99±13 |
Based on the gated-SPECT imaging, all the patients showed normal ejection fractions, but abnormal perfusion defects were observed in only some MCT patients. Specifically, there were fixed perfusion defects in 2/16 (12%) MCT patients and reversible perfusion defects in 7/16 (44%) MCT patients. On follow-up, only 13/16 (75%) MCT patients showed a worsening of the NYHA functional class (NYHA:1.8 ± 0.8 vs 2.9 ± 1; p = 0.0001). Only 7/16 (44%) MCT patients were NYHA class IV; 4/16 (25%) died from heart failure, and 3/16 (19%) died from heart failure with cancer progression. The clinical characteristics of the cancer patients, mechanisms of MCT cardiotoxic action [10], the gated-SPECT imaging and follow-up results are summarized in Table 2.
Table 2.
Patients clinical profiles
| Age/gender | Type of cancer | MCT before SPECT | E-EV | Basal NYHA | ADS | % EF (rest-gated-SPECT) | MCT after SPECT | Follow-up (months after SPECT) | NYHA/L-EV (on followup) |
|---|---|---|---|---|---|---|---|---|---|
| 52/M | non Hodgkin's lynphoma | Ca, Et-, Vinb, Ine, Epia, Ia | My-I | 1 | R | 43 | 0 | 31 | 3/0 |
| 57/M | Liver m. | Cab, Bevac, Oxb | 0 | 1 | R | 57 | Bevac, Myta, Cab, Fb | 27 | 4/HF-death with CP |
| 68/M | Colon with m. | Fb, Oxb, Bevac, Re | My-Isc | 1 | R | 50 | Cab, Fb | 31 | 1/0 |
| 41/FM | Breast wth m. | Aa, Trasta, Cab, Mytb, Mtxb, Vinorb, Re | AF | 2 | R | 63 | Trasta , Re Lape | 31 | 3/0 |
| 64/M | Colorectal | Bevac, Cab, Oxb | 0 | 1 | R | 52 | Ced | 26 | 3/0 |
| 56/FM | Colorectal | Bevac, Cab, Oxb | AF | 2 | R | 54 | Cab, Oxb | 7 | 4/AF-HF-death |
| 47/FM | Breast wth m. | Trasta, Bevac, Pxd, Ca, Vncb | 0 | 1 | 0 | 62 | Bevac | 31 | 1/0 |
| 87/M | Colon with m. | Ced, Cab, Cyta | 0 | 3 | 0 | 52 | Cab, Ced, Oxb | 18 | 4/HF-death with CP |
| 60/FM | Breast with m. | Trasta,Epid, Ca, Vinorb | SVT | 2 | 0 | 47 | Bevac, Cara, Gema | 31 | 3/0 |
| 49/FM | Breast | Epia, Ca, Dxa, Trast- | 0 | 2 | 0 | 64 | 0 | 31 | 2/0 |
| 37/FM | Breast | Epia, Ca, Dxa, Trasta, R*a | 0 | 1 | F | 65 | 0 | 26 | 2/0 |
| 82/M | Prostate, Gastric with m. | Pxd, Re | AF HF | 3 | 0 | 55 | 0 | 6 | 4/AF-HF-death |
| 73/M | Prostate, Gastric with m. | Dxa, Re | My-I (CAD) | 3 | 0 | 52 | 0 | 3 | 4/HF-death |
| 60/FM | Breast | Fb, Epia, Ca, Re | My-I | 1 | R | 66 | 0 | 31 | 1/0 |
| 38/M | Pancreatic with m. | Bevac, Cab | 0 | 2 | 0 | 60 | 0 | 6 | 4/HF-death with CP |
| 82/M | Lung and Colon with m. | Cab ,Oxb, Mytb, Fb, Ced | P E | 3 | F | 60 | 0 | 6 | 4/AF-HF-death |
Atrial fibrillation (AF), abnormal defects (ADS), coronary artery disease (CAD), early cardiovascular events (E-EV), late cardiovascular events (L-EV), female (FM), fixed ADS (F), heart failure (HF), New York Heart Association (NYHA), male (M), metastasis (m), myocardial infarction (My-I), myocardial ischemia (My-Isc), pulmonary embolism (PE), reversible ADS (R), supraventricular tachycardia (SVT), Adriamycin (A), Bevacizumab (Beva), Capecitabine (Ca), Carboplatin (Car), Cetuximab (CE), Cyclophosphamide (C), Cytarabine (Cyt), Docetaxel (Dx), Epirubicine (Epi), Etoposide (ET), Fluorouracil (F), Gemcitabine (Gem), Ifosfamide (I), Interferon (In), Lapatinib (Lap), Methotrexate (Mtx), Mytomycin (Myt), Oxaliplatin (Ox), Paclitaxel (Px), Radiotherapy (R), Trastuzumab (Trast), Vinorelbine (Vinor), Vincristine (Vnc), Cancer progression (CP).
Drugs with prevalent myocardial depression;
drugs with prevalent My-Isc;
drugs with prevalent hypertension
druge with prevelent hypotension
drugs with both myocardial depression and My-Isc.
When delayed and early cardiac MIBI uptake, ejection fraction, perfusion defects or cardiac MIBI % WR were evaluated with a logistic regression analysis, delayed cardiac MIBI uptake (Odds ratio = 1.7, 95% CI, 1.1 to 2.7, p<0.001) and early cardiac MIBI uptake (Odds ratio = 1.2, 95% CI, 1.1 to 1.5, p<0.02) were found to be significantly associated with cardiac mortality. The results did not change when the data were adjusted for age, total number of anti-cancer drugs used before nuclear imaging, duration of MCT, presence of early adverse effects or established cardiovascular risk factors (p > 0.05 for all). When the relationship between survival risk parameters and death was analyzed with ROC curves, delayed and early cardiac MIBI uptake showed a greater area under the ROC curve compared with the other parameters with a values of 0.93 (95% confidence interval from 0.80 to 1, p < 0.0001) and 85% (95% confidence interval from 0.68 to 0.95, p < 0.0001) and a criterion value of > 29 cpp (specificity = 86%, sensitivity = 91%) and and 42.8 ccp (specificity = 86%, sensitivity = 83%), respectively. The criterion value of the delayed cardiac uptake showed the highest average of sensitivity for screening the patients outcome and thus better stratified individuals with respect to mortality based on the Kaplan-Meier survival curve analysis (Figure 3).
Figure 3.
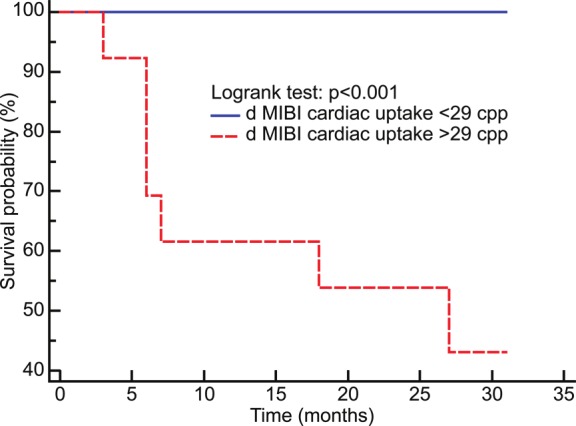
Kaplan-Meier curves; delayed (d) cardiac MIBI uptake values stratified individuals with respect to mortality.
Discussion
This study presents the concept that in chemotherapy patients, the cardiac MIBI kinetics are altered and may be a predictor of mortality from cardiac causes. The increased values of the early and delayed cardiac MIBI uptake in MCT patients compared with control patients were not incidental findings but were clearly related to the specific actions of MCT on myocardial metabolism. The liver MIBI kinetics were similar in the MCT and control patients, suggesting that MCT did not influence liver cellular function but only affected the metabolism of the myocytes. The similar grade of MIBI uptake shown at the metabolically inactive injection sites of the MCT and control patients further demonstrated that MCT showed exclusive action on the metabolic function of myocytes. In addition, MIBI uptake was not influenced by the weight or body size of the patients. In normally-functioning hearts, approximately 90% of the MIBI activity is associated with the mitochondria in an energydependent manner [11]. The tracer is taken up in the myocardium in proportion to blood flow and detects myocellular viability [12]. At equilibrium, MIBI is sequestered within mitochondria by the large negative transmembrane potentials [13]. Treatments that elicit hyperpolarization of cardiac mitochondrial membrane potentials induce thus a marked increase of cardiac MIBI uptake and retention [14]. Notably, the primary underlying mechanism of most anti-cancer drugs is to hyperpolarize mitochondrial membrane potentials [15-19]. These data, when translated to patients on MCT, suggest that the increased cardiac MIBI uptake compared with controls might be a consequence of mitochondrial membrane dysfunction due to the MCT treatment. The faster MIBI washout observed in MCT patients with respect to controls may indicate thus transient reversible mitochondrial membrane dysfunction as the “leaky” mitochondrial membrane lets more MIBI into the mitochondria and induces a faster MIBI “leak out” as well. Cardiac mitochondrial membrane hyperpolarization induces the opening of a nonspecific pore in the mitochondrial membrane that enables the free passage of molecules <1.5 kDa into the mitochondria [20]. The resulting uncoupling of oxidative phosphorylation leads to ATP depletion, inadequate energy production, arrhythmogenesis [21], myocardial dysfunction, and necrotic cell death [22]. Thus, the mitochondrial membrane increased permeability due to MCT may represent a preliminary phase that may precede irreversible mitochondrial damage resulting in decreased cardiac MIBI uptake and increased MIBI washout [23,24], severe myocardial dysfunction, and short-term mortality. In addition, myocardial damage and silent ischemia [25], which are direct consequences of myocyte and vascular damage from MCT [26], may induce arrhythmias [27] and transient LV dysfunction [28]. Such mechanisms thereby contribute to the worsened functional capacity and the high incidence of early adverse cardiotoxic effects observed in patients under study. However our study has some limitations due to the small patient sample. The effects of sequence, dosage, and the time interval between the administrations of the anticancer agents were not adequately considered [29], while coronary angiography was performed only in patients with acute coronary syndromes. This approach on a relatively small number of patients does not provide therefore definite clinical conclusions but suggests the basis for further studies.
Conclusion
This is, however, the first study that introduces the properties of cardiac MIBI kinesis as a method for the detection of pre-clinical cardiotoxicity in cancer patients on MCT. The evaluation of cardiac MIBI kinetics by planar imaging may provide additional informations concerning the myocardial metabolism of patients receiving MCT and suggest the need for an early cardiovascular protection [30] despite the presence of a normal ejection fraction.
References
- 1.Bassily MN, Wilson R, Pompei F, Burmistrov D. Cancer survival as a function of age at diagnosis: A study of the Surveillance, Epidemiology and End Results database. Cancer Epidemiol. 2010;34:667–681. doi: 10.1016/j.canep.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Torrisi JM, Schwartz LH, Gollub MJ, Ginsberg MS, Bosl GJ, Hricak H. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology. 2011;258:41–56. doi: 10.1148/radiol.10092129. [DOI] [PubMed] [Google Scholar]
- 3.Frezza C, Gottlieb E. Mitochondria in cancer: Not just innocent bystanders. Semin Cancer Biol. 2009;19:411. doi: 10.1016/j.semcancer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Crane P, Laliberté R, Heminway S, Thoolen M, Orlandi C. Effect of mitochondrial viability and metabolism on technetium-99m-sestamibi myocardial retention. Eur J Nucl Med. 1993;20:20–25. doi: 10.1007/BF02261241. [DOI] [PubMed] [Google Scholar]
- 5.Fukushima K, Momose M, Kondo C, Higuchi T, Kusakabe K, Hagiwara N. Myocardial 99mTcsestamibi extraction and washout in hypertensive heart failure using an isolated rat heart. Nucl Med Biol. 2010;37:1005–1012. doi: 10.1016/j.nucmedbio.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson JO, Polovich M, McNiff KK, Lefebvre KB, Cummings C, Galioto M, Bonelli KR, McCorkle MR; American Society of Clinical Oncology; Oncology Nursing Society. American Society Of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. J. Clin. Oncol. 2009;27:5469–5475. doi: 10.1200/JCO.2009.25.1264. [DOI] [PubMed] [Google Scholar]
- 7.The imaging market guide. USA Edition 1992-2008. Malvern, PA: rlington Medical Resources, Inc; [Google Scholar]
- 8.Matsuo S, Nakae I, Tsutamoto T, Okamoto N, Horie M. A novel clinical indicator using Tc-99m sestamibi for evaluating cardiac mitochondrial function in patients with cardiomyopathies. J Nucl Cardiol. 2007;14:215–220. doi: 10.1016/j.nuclcard.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Berman DS, Kang X, Gransar H, Gerlach J, Friedman JD, Hayes SW, Thomson LE, Hachamovitch R, Shaw LJ, Slomka PJ, Yang LD, Germano G. Quantitative assessment of myocardial perfusion abnormality on SPECT myocardial perfusion imaging is more reproducible than expert visual analysis. J Nucl Cardiol. 2009;16:45–53. doi: 10.1007/s12350-008-9018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, Durand JB, Gibbs H, Zafarmand AA, Ewer MS. Cardiovascular complications of cancer therapy diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho PA, Chiu ML, Kronauge JF, Kawamura M, Jones AG, Holman BL, Piwnica-Worms D. Subcellular distribution and analysis of technetium-99m-MIBI in isolated perfused rat hearts. J Nucl Med. 1992;33:1516–1522. [PubMed] [Google Scholar]
- 12.Beller GA, Watson DD. Physiological basis of myocardial perfusion imaging with the technetium 99m agents. Semin Nucl Med. 1991;21:173–181. doi: 10.1016/s0001-2998(05)80038-8. [DOI] [PubMed] [Google Scholar]
- 13.Piwnica-Worms D, Kronauge JF, Chiu ML. Uptake and retention of hexakis (2-methoxyisobutylisonitrile) technetium (I) in cultured chick myocardial cells. Mitochondrial and plasma membrane potential dependence. Circulation. 1990;82:1826–1838. doi: 10.1161/01.cir.82.5.1826. [DOI] [PubMed] [Google Scholar]
- 14.Piwnica-Worms D, Kronauge JF, Chiu ML. Enhancement by tetraphenylborate of technetium-99m-MIBI uptake kinetics and accumulation in cultured chick myocardial cells. J Nucl Med. 1991;32:1992–1999. [PubMed] [Google Scholar]
- 15.Montaigne D, Marechal X, Preau S, Baccouch R, Modine T, Fayad G, Lancel S, Neviere R. Doxorubicin induces mitochondrial permeability transition and contractile dysfunction in the human myocardium. Mitochondrion. 2011;11:22–26. doi: 10.1016/j.mito.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Walliser S, Redmann K. Effect of 5-fluorouracil and thymidine on the transmembrane potential and zeta potential of HeLa cells. Cancer Res. 1978;38:3555–3559. [PubMed] [Google Scholar]
- 17.Ma H, Jones KR, Guo R, Xu P, Shen Y, Ren J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol. 2010;37:460–465. doi: 10.1111/j.1440-1681.2009.05323.x. [DOI] [PubMed] [Google Scholar]
- 18.al-Nasser IA. In vivo prevention of cyclophosphamide-induced Ca2+ dependent damage of rat heart and liver mitochondria by cyclosporin A. Comp Biochem Physiol A Mol Integr Physiol. 1998;121:209–214. doi: 10.1016/s1095-6433(98)10135-6. [DOI] [PubMed] [Google Scholar]
- 19.Grazette LP, Boecker W, Matsui T, Semigran M, Force TL, Hajjar RJ, Rosenzweig A. Inhibition of ErbB2 causes mitochondrial dysfunction in cardiomyocytes: implications for herceptin-induced cardiomyopathy. J Am Coll Cardiol. 2004;44:2231–2238. doi: 10.1016/j.jacc.2004.08.066. [DOI] [PubMed] [Google Scholar]
- 20.Halestrap AP. What is the mitochondrial permeability transition pore? Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 21.Brown DA, O'Rourke B. Cardiac mitochondria and arrhythmias. Cardiovasc Res. 2010;88:241–249. doi: 10.1093/cvr/cvq231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baines CP. The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatr Cardiol. 2011;32:258–262. doi: 10.1007/s00246-010-9880-9. [DOI] [PubMed] [Google Scholar]
- 23.Kumita S, Seino Y, Cho K, Nakajo H, Toba M, Fukushima Y, Okamoto N, Takano T, Kumazaki T. Assessment of myocardial washout of Tc-99m-sestamibi in patients with chronic heart failure: comparison with normal control. Ann Nucl Med. 2002;16:237–242. doi: 10.1007/BF03000101. [DOI] [PubMed] [Google Scholar]
- 24.Isobe S, Ohshima S, Unno K, Izawa H, Kato K, Noda A, Hirashiki A, Murohara T. Relation of 99m Tc-sestamibi washout with myocardial properties in patients with hypertrophic cardiomyopathy. J Nucl Cardiol. 2010;17:1082–1090. doi: 10.1007/s12350-010-9266-7. [DOI] [PubMed] [Google Scholar]
- 25.Daher IN, Yeh ET. Vascular complications of selected cancer therapies. Nat Clin Pract Cardiovasc Med. 2008;5:797–805. doi: 10.1038/ncpcardio1375. [DOI] [PubMed] [Google Scholar]
- 26.Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14–25. doi: 10.1093/jnci/djp440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myerburg RJ, Kessler KM, Mallon SM, Cox MM, deMarchena E, Interian A Jr, Castellanos A. Life-threatening ventricular arrhythmias in patients with silent myocardial ischemia due to coronary-artery spasm. N Engl J Med. 1992;326:1451–1455. doi: 10.1056/NEJM199205283262202. [DOI] [PubMed] [Google Scholar]
- 28.Opie LH. The multifarious spectrum of ischemic left ventricular dysfunction: relevance of new ischemic syndromes. J Mol Cell Cardiol. 1996;28:2403–2414. doi: 10.1006/jmcc.1996.0233. [DOI] [PubMed] [Google Scholar]
- 29.Saad SY, Najjar TA, Alashari M. Cardiotoxicity of doxorubicin/paclitaxel combination in rats: effect of sequence and timing of administration. J BiochemMolToxicol. 2004;18:78–86. doi: 10.1002/jbt.20012. [DOI] [PubMed] [Google Scholar]
- 30.Debatin KM, Poncet D, Kroemer G. Chemotherapy: targeting the mitochondrial cell death pathway. Detecting MMP might thus be useful for detecting chemotherapy responses in vivo. Oncogene. 2002;21:8786–8803. doi: 10.1038/sj.onc.1206039. [DOI] [PubMed] [Google Scholar]


