Abstract
Abstract:
The allergy cascade presents widespread inflammatory and proinflammatory activation, robust cytokine and chemokine signaling, and heterogeneous immune and endothelial responses that lead ultimately to the manifestations of allergic reaction. Histamine, a small peptide with inherent vasoactive properties, is released from granules contained within mast cells, basophils, lymphocytes, and other reservoirs and interacts with histamine receptors to regulate numerous cellular functions involved in allergic inflammation and immune modulation. Of the known histamine receptors, the H1-receptor is most clearly associated with potentiation of proinflammatory immune cell activity and enhanced effector function and is the prime focus of suppressive therapy. Second-generation oral H1-antihistamines, such as cetirizine, desloratadine, fexofenadine, levocetirizine, and loratadine, are mainstays of allergy treatment, acting as highly specific, long-acting H1-receptor agonists at its unique receptor. The ongoing identification of immune effector cells and mediators involved in the allergic cascade indicates that further research is necessary to define the role of antihistamines such as desloratadine in anti-inflammatory therapy.
Key Words: allergic rhinitis, antihistamine, anti-inflammatory, desloratadine, second-generation antihistamines, urticaria
INTRODUCTION
Histamine, one of the most intensively studied molecules in medicine,1 is a key mediator in allergic rhinitis (AR) and urticaria. Interacting with a unique group of membrane-bound receptors widely distributed across immune cell subtypes, histamine participates in intricate bidirectional messaging between cytokines and inflammatory cells or their precursors, facilitates migration of cells to inflammatory sites, stimulates lymphocyte activity, modulates aspects of eosinophil, neutrophil and mast cell behavior (Fig. 1),1–4 and is directly implicated in the generation of cardinal allergic symptoms such as rhinorrhea; sneezing; congestion; nasal, ocular, and dermal pruritus; hives; and flushing.5
FIGURE 1.
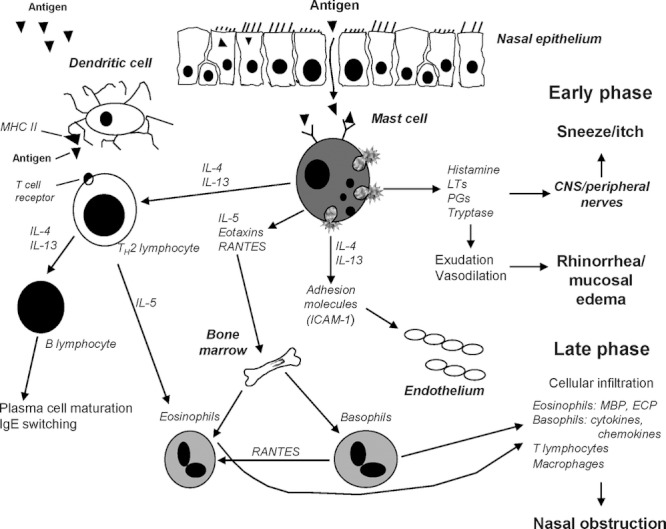
The allergic cascade. Mast cell mediators, including cytokines, cause degranulation and contribute to the bidirectional messaging with other inflammatory cells or their precursors, lymphocyte activity, and migration of immune cells to inflammatory sites. CNS, central nervous system; ECP, eosinophil cationic protein; ICAM, intracellular adhesion molecule; Ig, immunoglobulin, IL, interleukin; LT, leukotriene; MBP, mannose-binding protein; MHC II, major histocompatability complex; PGs, prostaglandins. Reprinted with permission from Baena-Cagnani et al.4
The H1-histamine receptor is most clearly associated with modulation of proinflammatory immune cell activity,6,7 and its interaction with histamine is the prime focus of suppressive therapy for AR and urticaria with second-generation H1-antihistamines such as cetirizine, desloratadine, ebastine, fexofenadine, levocetirizine, loratadine, and rupatadine.
As a class, these antihistamines are consistently effective for symptom relief in histamine-mediated diseases. When used at recommended doses, second-generation antihistamines are essentially devoid of undesirable central nervous system (CNS) effects such as somnolence, and are much less likely to cause other undesirable anticholinergic side effects characteristic of first-generation antihistamines.8 Current management guidelines recommend second-generation antihistamines for first-line therapy for AR and urticaria.9,10
Differences exist in the pharmacology of individual second-generation antihistamines (Table 1)8,11–13 and, possibly, in their individual ability to suppress proinflammatory mediators associated with an unfolding allergic response.14 Some anti-inflammatory effects of antihistamines seem to require initial interaction with the histamine receptor while others are receptor-independent.15 Further study is needed to determine whether these differences in anti-inflammatory pharmacology translate into clinically meaningful effects.8
TABLE 1.
Pharmacology of Select Second-Generation Antihistamines(8,11–13)
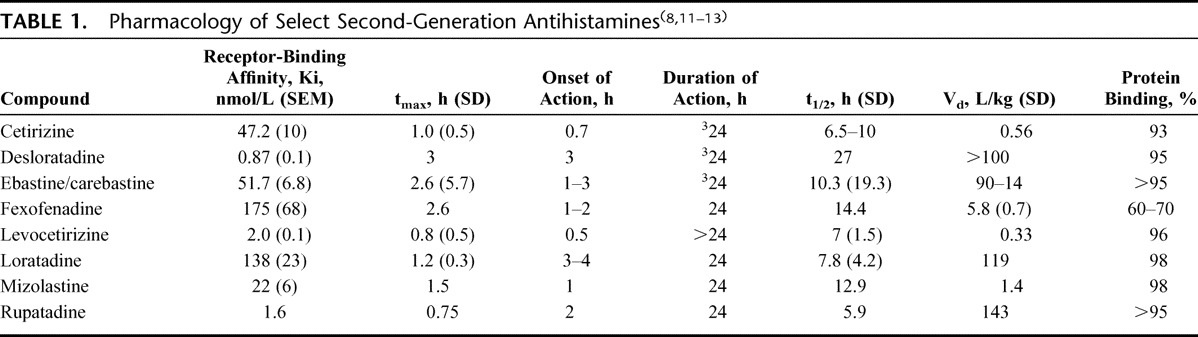
Desloratadine, the active metabolite of loratadine, is a second-generation oral antihistamine with proven efficacy in randomized, controlled clinical trials, and a safety and tolerability profile similar to placebo.16–27 In the European Union, desloratadine is indicated for the treatment of intermittent and persistent AR and urticaria in adults and children aged ≥1 year.28 Desloratadine is approved in the United States for the treatment of seasonal AR in adults and children aged ≥2 years and perennial AR and chronic idiopathic urticaria (CIU) in adults and children aged ≥6 months.29 In vitro studies, studies in animal models, and in vivo investigations demonstrate that desloratadine, similar to antihistamines such as levocetirizine (the active enantiomer of cetirizine) and others, inhibits a range of inflammatory mediators in addition to exerting potent H1-receptor antagonism.12 The present review will report recently available information expanding the antihistaminic, anti-inflammatory, and antiallergic profile of desloratadine.
THE H1-HISTAMINE RECEPTOR
Four specialized, widely distributed receptors (designated H1, H2, H3, H4) mediate the effects of histamine.1 The local concentration of histamine and predominant type of histamine receptor undergoing activation determines the type of effector response that is elicited.6,8 Most cells involved in inflammatory reactions express H1, H2, and H4 subtypes,6 with the H1-receptor playing a major role in potentiation of proinflammatory immune cell activity and effector responses fundamental to an allergic reaction; the H2-receptor, in contrast, appears to suppress inflammatory and effector functions, while data regarding the role of the H4-receptor in immune response are limited.1,3
The H1-receptor is a transmembrane protein belonging to the G-protein coupled receptor family. Signal transduction from the extracellular to the intracellular environment occurs as the GCPR becomes activated after binding of a specific ligand or agonist. A subunit of the G-protein subsequently dissociates and affects intracellular messaging including downstream signaling accomplished through various intermediaries such as cyclic AMP, cyclic GMP, calcium, and nuclear factor kappa B (NF-κB), a ubiquitous transcription factor thought to play an important role in immune-cell chemotaxis, proinflammatory cytokine production, expression of cell adhesion molecules, and other allergic and inflammatory conditions.1,8,12,30–32
The classic model of receptor activation requires binding by a specific ligand, or agonist. Advances in understanding of histamine receptor behavior have established that histamine receptors can exhibit inherent, spontaneous activity (“constitutive activity”) that is independent of receptor occupancy by an agonist. A spontaneously activated histamine receptor interacts with its intracellular effector system through its typical intermediary, and elicits a downstream event even in the absence of histamine binding.15 The concept of constitutive activity has led to a reclassification of drugs acting at the H1-receptor. Antihistamines that combine with the inactive form of the receptor can be considered “inverse agonists,” stabilizing receptor behavior in the inactive state and reducing the population of receptors exhibiting constitutive activity.15,33,34 For example, the H1-receptor promotes NF-κB in both a constitutive and agonist-dependent manner and all clinically available H1-antihistamines inhibit constitutive H1-receptor-mediated NF-κB production; an inverse H2-agonist or an H3-antagonist have no effect15,35 (Fig. 2). Ligands having no effect on basal levels of receptor constitutive activity but that interfere with binding of agonists are considered “neutral antagonists” under this scheme. Importantly, because antihistamines can theoretically behave as inverse agonists or neutral antagonists, they are more properly described as H1-antihistamines rather than H1-receptor antagonists.15
FIGURE 2.
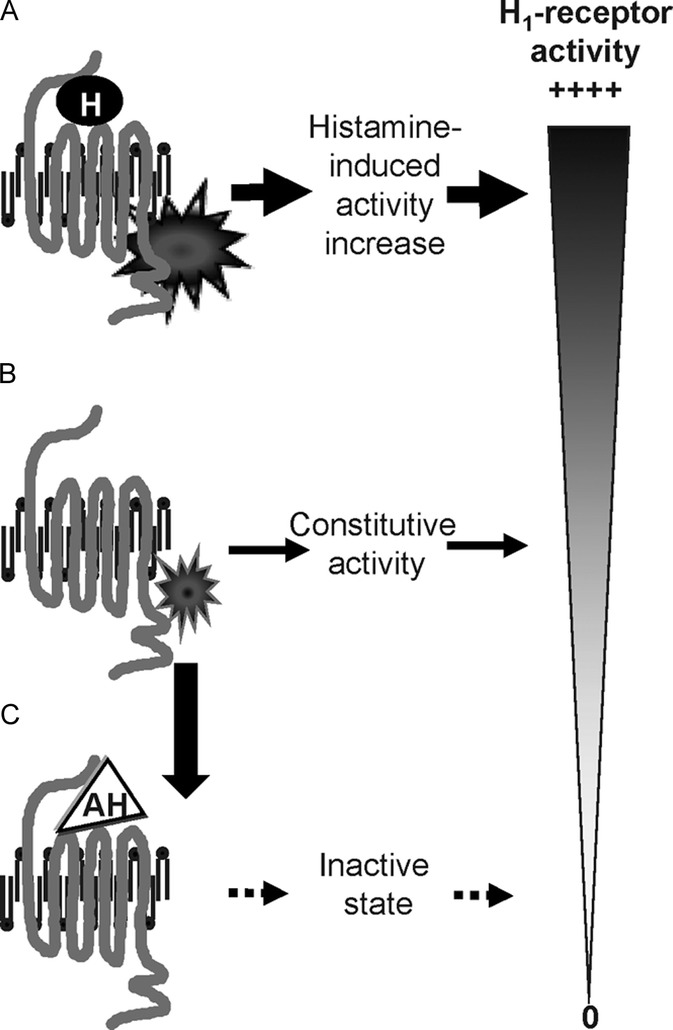
H1-receptors may be activated by an antagonist, such as histamine (A) or exhibit spontaneous basal, or constitutive, activity in the absence of ligand binding (B). Antihistamines repress both histamine-induced and constitutive activity, eventually restoring the receptor to an inactive state (C). AH, antihistamine; H, histamine.
Desloratadine: Antihistaminic, Anti-Inflammatory, and Antiallergic Effects
Affinity for the H1-receptor
Desloratadine binds avidly and noncompetitively to a recombinant human H1-receptor, displaying 52, 57, 194, and 153 times more potency for the interaction than cetirizine, ebastine, fexofenadine, and loratadine, respectively (Table 1); measured change in histamine-induced intracellular calcium was the effector end point used in the assay.36 Once desloratadine is bound, disassociation from the receptor is slow; only 37% of desloratadine is unbound at 6 hours, suggesting pseudo-irreversibility and supporting an extended duration of action.36
As a second-generation antihistamine, desloratadine demonstrated inverse agonism, reducing downstream messaging by spontaneously active receptors. In one study, desloratadine effectively inhibited downstream signaling of a constitutively active human H1-receptor associated with NF-κB formation, reducing basal NF-κB activity to a greater extent than did equivalent concentrations of cetirizine, fexofenadine, loratadine, or pyrilamine.37 In addition, desloratadine was more potent than comparators in blocking the rise of NF-κB after activation of the receptor by exposure to histamine.37
Effects on Immune Cells: Eosinophil Activation, Migration, and Survival
Eosinophils, key effector cells in the allergic response, are recruited from the circulation to sites of inflammatory activity where they participate in immune reactions and secrete an array of preformed cytotoxic cationic proteins (major basic protein, cationic protein, peroxidase, neurotoxin protein). Eosinophils also produce cytokines, chemokines, leukotrienes, and neuromodulators.38 Desloratadine may exert effects on eosinophil chemoattractants, precursors, activation, and survival.
Desloratadine reduces the expression of NF-κB, a known inducer of RANTES (regulated upon activation, normal T-cell expressed and secreted), a principal chemoattractant for eosinophils, monocytes, and t-lymphocytes. RANTES promotes eosinophil activation and the release of histamine from basophils.37,39 Desloratadine inhibited the release of RANTES by nasal polyp epithelial cell lines in response to tumor necrosis factor (TNF),40 eosinophil cationic protein, and activated mast cells; this inhibition was also reflected in diminished production of tryptase and leukotriene C4.32,41 In an investigation enrolling atopic individuals with persistent asthma randomized to multiweek treatment with desloratadine or an oral steroid, both medications significantly reduced the expression of mRNA specific for chemokines involved in T-cell signaling and eosinophil or basophil activation, including RANTES, macrophage inflammatory protein (MIP)-1 α, and MIP-1 β.39,42
The number of eosinophil/basophil progenitors (Eo/B) in the peripheral circulation typically falls in atopic individuals as these cells migrate to sites of inflammation through chemoattraction, vascular adhesion, and extravasation.43 In a randomized, placebo-controlled study of 45 subjects with symptomatic, seasonal AR treated with desloratadine (20 mg daily) or placebo, those receiving desloratadine demonstrated a significantly greater increase in peripheral blood Eo/B progenitors during the first 2 weeks of treatment compared with subjects given placebo. Further, a statistically significant decrease in nasal lavage eotaxin was found in the desloratadine group compared with placebo, which may support the concept that desloratadine blocks the migration of these cells from circulation to sites of inflammation within nasal tissue.43 In a study assessing eosinophil survival at the site of upper airway inflammation, the survival of peripheral blood eosinophils incubated with human epithelial cell conditioned media from nasal mucosa or nasal polyp tissue was reduced in a dose-dependent manner after preincubation with desloratadine.44
Effects on Immune Cells: Mast Cells and Basophils
Antihistamines relieve symptoms of AR and urticaria primarily by competing with histamine at the H1-receptor; emerging evidence indicates that antihistamines may also inhibit mast cell degranulation and subsequent histamine release.45,46
Human mast and basophilic cells exposed to desloratadine show reduced production of cytokines central to inflammatory responses.14,46–48 In one report, desloratadine reduced phorbol 12-myristate 13-acetate secretagogue-stimulated mast cell release of interleukin (IL)-3, IL-6, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) by 32.1, 32.6, 64.5, and 27.8%, respectively; reductions displayed dose-dependency, optimal effects were obtained at concentrations easily achieved in clinical settings and were in some instances comparable with suppression obtained with dexamethasone and always significantly better than cetirizine (P < 0.05)14 (Fig. 3).
FIGURE 3.
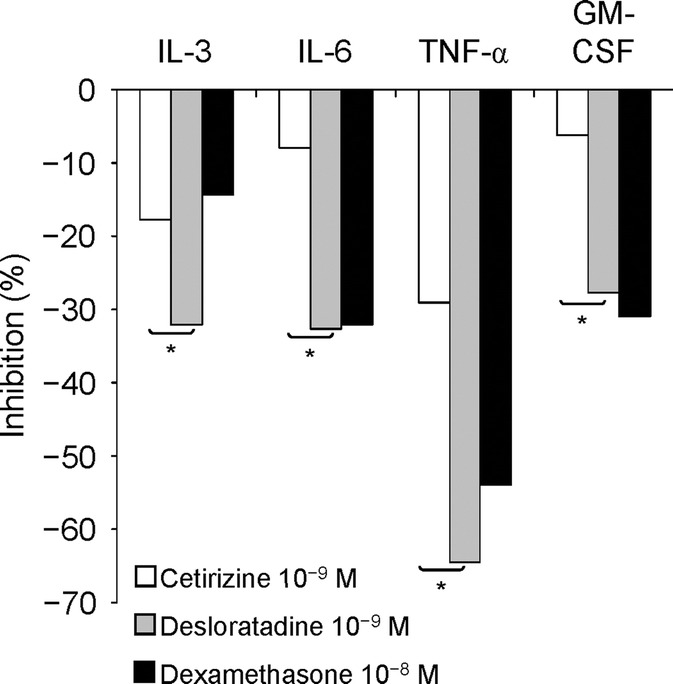
Inhibition of cytokine release from human mast cells with cetirizine, desloratadine, and dexamethasone. *P < 0.05.14 Experiments were conducted by preincubating human leukemic mast cells with the H1-blockers cetirizine and desloratadine and the H-2 blocker dexamethasone for 1 hour, followed by a 24-hour coincubation with phorbol myristate acetate (25 ng/mL) and the calcium ionophore A 23187 (2.5 × 10−7 M). Data are expressed as means of at least 4 experiments. GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; TNF-α, tumor necrosis factor-α.
Desloratadine demonstrated dose-dependent inhibition of CD107a (a marker of mast cell degranulation) expression on human mast cells after stimulation with anti-IgE, calcium ionophore, or substance P. At the highest desloratadine concentration (10−4 M), the inhibitory effect for anti-IgE, substance P, and calcium ionophore (50.7, 48.0, and 26.7% inhibition; P < 0.05 vs. 100% stimulation for each test substance in the absence of desloratadine). Desloratadine at the highest concentration also significantly inhibited histamine release by mast cells after stimulation with each of the 3 test substances (42.6, 53.7, and 39.9% inhibition; P < 0.01 vs. 100% stimulation for each test substance in the absence of desloratadine).48
IL-4 is a B-lymphocyte growth factor and a major mediator of the allergic response, implicated in antigen presentation and cytokine production.49 Human basophils are a major source of IL-4 produced in experimental immune cell cultures.50 In one investigation, basophil-enriched suspensions were incubated with various concentrations of desloratadine for 15 minutes before stimulation with anti-IgE antibody, calcium ionophore, IL-3, or phorbol ester. Notably, desloratadine was nearly 6 to 7 times more potent in preventing the secretion of IL-4 and IL-13 induced by anti-IgE than it was at inhibiting the release of histamine and leukotriene C4. After desloratadine pretreatment, IL-4 mRNA was inhibited by as much as 80%.50 These results are consistent with an earlier evaluation of the inhibitory effects of desloratadine and steroids (budesonide and dexamethasone) on mast cell release of IL-4, IL-6, IL-8, GM-CSF, and TNF-α after secretagogue stimulation. Desloratadine demonstrated less cytokine inhibition compared with a glucocorticoid, but statistically significant (P < 0.01) inhibition of IL-4 was noted at all desloratadine concentrations (10−6 to 10−10 M) and time points (6, 12, and 24 hours). Inhibition of TNF-α was statistically significant (P < 0.05) at concentrations of 10−6 M (all time points) and 10−8 M (6 and 12 hours), whereas inhibition of IL-6 and GM-CSF was statistically significant (P < 0.05) at one concentration (10−6 M and 10−8 M, respectively) and at a single time point (6 hours).51
Effects on Immune Cell Adhesion
Desloratadine has effects on the adhesion of activated immune cells to endothelial and epithelial tissues. Desloratadine inhibits the in vitro histamine-induced expression of P-selectin (implicated in the adhesion and migration of neutrophils and eosinophils) and leads to decreased production of IL-6, IL-8, and IL-8 mRNA.52 Nasal epithelial cells incubated with desloratadine for 24 hours demonstrate significantly reduced expression of intercellular adhesion molecule (ICAM)-1 (implicated in nasal epithelial cell activation) after histamine exposure.53
Effects on Inflammatory Mediators
In the first study to evaluate the potential anti-inflammatory activities of desloratadine on human epidermal cells, keratinocyte cultures from normal skin were activated by interferon (IFN)-γ in the absence or presence of desloratadine and evaluated for the release of RANTES, CXCL8, CCL17/TARC, and CXCL10. Desloratadine dose-dependently inhibited the constitutive and induced release of RANTES, CXCL8, and CXCL10. In addition, supernatant from keratinocytes was evaluated at 48 hours for its capacity to attract immune cell types. Desloratadine dose-dependently reduced the migration of T-helper (TH)1 and TH2 cells toward IFN-γ–stimulated keratinocytes and inhibited the release of constitutive and IFN-γ–induced chemoattractants for human neutrophils and eosinophils. RANTES-associated eosinophil trafficking demonstrating the most significant reduction. In this study, concentrations of desloratadine (1–100 μM) required for inhibition of cytokines were higher than those considered achievable in plasma at a typical dose of 10 mg/d.54
In Vivo Investigation
Cold urticaria: Dose-dependency of anti-inflammatory and antihistaminic effects
While in vitro findings strongly suggest that desloratadine possesses an anti-inflammatory capability directed against important cytokines, the clinical relevance of these findings using standard dosing remains unclear. Many of the reported anti-inflammatory effects of desloratadine are seen with higher than standard doses in experimental settings.44,48,49,54 In contrast, an open, uncontrolled in vivo investigation of standard-dose desloratadine given to subjects with seasonal AR and concomitant asthma failed to document a significant reduction in any examined mediator (IL-4, IL-8, IL-10, tumor growth factor-β) after 4 weeks of treatment.49 In a prospective, randomized, double-blind, crossover trial, 30 subjects with acquired cold urticaria (ACU), a disease in which clinical features are because of release of mast cell mediators in response to cold, received placebo, desloratadine 5 mg, or desloratadine 20 mg every day, each for 7 days, separated by 14-day washout periods. After cold provocation, urticarial reactions were assessed with digital 3-dimensional imaging and thermography; critical temperature and critical stimulation time thresholds were measured. High-dose desloratadine significantly improved objective signs of ACU and significantly reduced ACU lesion severity versus desloratadine 5 mg without any adverse safety or tolerability concerns.17 These results strongly suggest that the antihistaminic and anti-inflammatory effects of desloratadine increase in a clinically relevant fashion with dose escalation, and support current guidelines recommending desloratadine dosage escalation for treatment of ACU.17
DISCUSSION
H1-receptors are expressed on many cell types, including mast cells, basophils, dendritic cells, endothelial cells, and smooth muscle cells.2,6 Their stimulation by histamine produces the cardinal symptoms of an allergic response. The presence of histamine up-regulates the population of histamine receptors, and it is likely that up-regulation is a continuous process while histamine is present. Receptor up-regulation is blocked experimentally by the presence of antihistamines.7 Further, H1-receptors can demonstrate spontaneous, constitutive signaling even in the absence of histamine stimulation. H1-receptor antagonists effectively mitigate allergy symptoms and reduce both constitutive and histamine-stimulated receptor signaling.
Desloratadine, a second-generation H1-receptor antagonist, has proven clinical efficacy across a range of histamine-mediated conditions and a safety and tolerability profile similar to placebo.16–27 It has the longest half-life of any of the second-generation antihistamines8 and binds to the H1-receptor with the highest affinity, disassociates slowly, displays noncompetitive antagonism and inverse agonism, and effectively modulates histamine-mediated allergic phenomena associated with AR and urticaria. Desloratadine is nonsedating in adults and free of muscarinic side effects.8,55–57
It should be noted that other second-generation antihistamines exhibit antiallergic, antihistaminic, and anti-inflammatory effects similar to desloratadine's. In a series of pilot studies in subjects with persistent allergic rhinitis (PER), Ciprandi et al investigated the effects of several of the newer second-generation antihistamines at indicated doses on nasal congestion, nasal airflow, and allergic inflammation. Rupatadine significantly improved nasal congestion (P = 0.0015) and increased nasal airflow (P = 0.0025) in 20 subjects with PER.58 Ebastine treatment in 20 patients with PER also significantly improved nasal congestion (P = 0.0025) and nasal airflow (P = 0.0001).59 Desloratadine and levocetirizine improved nasal airflow in a 30 patients with PER (P = ns and P < 0.001, respectively).60 Levocetirizine also significantly improved nasal airflow (P < 0.001) in a study of 40 patients with PER.61
Some limitations of this review should also be mentioned. The volume of distribution (Vd) of desloratadine of >100 L/kg is much greater than that of other second-generation antihistamines, which may counterbalance its higher receptor affinity. Vd is the apparent volume in which a determined dose of the drug distributes itself in the body at equilbrium. However, receptor occupancy is due to several factors other than volume of distribution: total plasma concentration of the drug, which relates to dose of the agent, its bioavailablility, and its plasma half-life. Level of receptor occupancy also depends on the degree of plasma protein binding, or the free plasma concentration available to the receptor;62 percentage of protein binding for desloratadine is comparable with other second-generation agents (Table 1). Finally, our search revealed no published randomized controlled studies with in vivo data demonstrating the anti-inflammatory response in AR for desloratadine or other second-generation antihistamines. More well-designed studies are needed to determine the effects of these second-generation agents on the inflammatory response in AR patients.
CONCLUSION
The biochemical and effector pathways provoked by the allergic response offer many potential targets and mechanisms by which desloratadine may modulate histamine-receptor activity, down-regulate inflammation cytokines and chemokines, or stimulate inflammatory cell migration and survival. In vitro data support anti-inflammatory effects of desloratadine on inflammatory cell function and mediator release. However, these studies typically use concentrations of desloratadine that are higher than what is achieved clinically at currently recommended dosing. Dose-dependent inhibition of the inflammatory response seen in vitro suggests that dose escalation of desloratadine may achieve a more potent anti-inflammatory response. Accumulating information suggests that desloratadine can modulate aspects of inflammation through mechanisms other than H1-histamine receptor blockade. Possible mechanisms await further investigation.
ACKNOWLEDGMENTS
Financial support for this review was provided by Schering-Plough Corp., now Merck & Co., Inc., Whitehouse Station, NJ. Editorial assistance was provided by Patricia C. Abramo and Karl Torbey, MD, of AdelphiEden Health Communications. This assistance was funded by Schering-Plough Corp.
REFERENCES
- 1.Akdis CA, Simons FER. Histamine receptors are hot in immunopharmacology. Eur J Pharmacol. 2006;533:69–76 [DOI] [PubMed] [Google Scholar]
- 2.Akdis CA, Jutel M, Akdis M. Regulatory effects of histamine and histamine receptor expression in human allergic immune responses. Chem Immunol Allergy. 2008;94:67–82 [DOI] [PubMed] [Google Scholar]
- 3.Schneider E, Rolli-Derkinderen M, Arock M, Dy M. Trends in histamine research: new functions during immune responses and hematopoiesis. Trends Immunol. 2002;23:255–263 [DOI] [PubMed] [Google Scholar]
- 4.Baena-Cagnani CE. Desloratadine activity in concurrent seasonal allergic rhinitis and asthma. Allergy. 2001;56:21–27 [DOI] [PubMed] [Google Scholar]
- 5.Zuberbier T, Asero R, Bindslev-Jensen C. EAACI/GA2LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009;64:1418–1426 [DOI] [PubMed] [Google Scholar]
- 6.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53 [DOI] [PubMed] [Google Scholar]
- 7.Das AK, Yoshimura S, Mishima R, Fujimoto K, Mizuguchi H, et al. Stimulation of histamine H1 receptor up-regulates histamine H1 receptor itself through activation of receptor gene transcription. J Pharmacol Sci. 2007;103:374–382 [DOI] [PubMed] [Google Scholar]
- 8.Simons FER. Advances in H1-antihistamines. N Engl J Med. 2004;351:2203–2217 [DOI] [PubMed] [Google Scholar]
- 9.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen). Allergy. 2008;63(Suppl 86):8–160 [DOI] [PubMed] [Google Scholar]
- 10.Zuberbier T, Asero R, Bindslev-Jensen C. EAACI/GA2LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–1443 [DOI] [PubMed] [Google Scholar]
- 11.del Cuvillo A, Mullol J, Bartra J, Dávila I, Jáuregui I, et al. Comparative pharmacology of the H1 antihistamines. J Investig Allergol Clin Immunol. 2006;16(Suppl 1):3–12 [PubMed] [Google Scholar]
- 12.Scadding G. Predicting and establishing the clinical efficacy of a histamine H1- receptor antagonist: desloratadine, the model paradigm. Clin Drug Invest. 2005;25:153–164 [DOI] [PubMed] [Google Scholar]
- 13.Simons FE. Comparative pharmacology of H1 antihistamines: clinical relevance. Am J Med. 2002;113(Suppl 9A):38S–46S [DOI] [PubMed] [Google Scholar]
- 14.Lippert U, Möller A, Welker P, Artuc M, Henz BM. Inhibition of cytokine secretion from human leukemic mast cells and basophils by H1- and H2-receptor antagonists. Exp Dermatol. 2000;9:118–124 [DOI] [PubMed] [Google Scholar]
- 15.Leurs R, Church MK, Taglialatela M. H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects. Clin Exp Allergy. 2002;32:489–498 [DOI] [PubMed] [Google Scholar]
- 16.Bousquet J, Bachert C, Canonica GW, Mullol J, Van Cauwenberge P, et al. , ACCEPT-1 Study Group Efficacy of desloratadine in intermittent allergic rhinitis: a GA2LEN study. Allergy. 2009;64:1516–1523 [DOI] [PubMed] [Google Scholar]
- 17.Siebenhaar F, Degener F, Zuberbier T, Martus P, Maurer M. High-dose desloratadine decreases wheal volume and improves cold provocation thresholds compared with standard-dose treatment in patients with acquired cold urticaria: a randomized, placebo-controlled, crossover study. J Allergy Clin Immunol. 2009;123:672–679 [DOI] [PubMed] [Google Scholar]
- 18.Zuberbier T, Canonica GW. Desloratadine significantly decreases total symptoms scores in subjects with persistent allergic rhinitis: ACCEPT-2 study in collaboration with GA2LEN. Ann Allergy Asthma Immunol. 2009;102:A119 [Google Scholar]
- 19.Canonica GW, Tarantini F, Compalati E, Penagos M. Efficacy of desloratadine in the treatment of allergic rhinitis: a meta-analysis of randomized, double-blind, controlled trials. Allergy. 2007;62:359–366 [DOI] [PubMed] [Google Scholar]
- 20.Ortonne J-P, Grob J-J, Auquier P, Dreyfus I. Efficacy and safety of desloratadine in adults with chronic idiopathic urticaria: a randomized, double-blind, placebo-controlled, multicenter trial. Am J Clin Dermatol. 2007;8:37–42 [DOI] [PubMed] [Google Scholar]
- 21.Pradalier A, Neukirch C, Dreyfus I, Devillier P. Desloratadine improves quality of life and symptom severity in patients with allergic rhinitis. Allergy. 2007;62:1331–1334 [DOI] [PubMed] [Google Scholar]
- 22.Berger WE, Lumry WR, Meltzer EO, Pearlman DS. Efficacy of desloratadine, 5 mg, compared with fexofenadine, 180 mg, in patients with symptomatic seasonal allergic rhinitis. Allergy Asthma Proc. 2006;27:214–223 [DOI] [PubMed] [Google Scholar]
- 23.Kim K, Sussman G, Hébert J, Lumry William, Lutsky B, Gates D. Desloratadine therapy for symptoms associated with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2006;96:460–465 [DOI] [PubMed] [Google Scholar]
- 24.Meltzer EO, Jalowayski AA, Vogt K, Iezzoni D, Harris AG. Effect of desloratadine therapy on symptom scores and measures of nasal patency in seasonal allergic rhinitis: results of a single-center, placebo-controlled trial. Ann Allergy Asthma Immunol. 2006;96:363–368 [DOI] [PubMed] [Google Scholar]
- 25.Monroe E, Finn A, Patel P, Guerrero R, Ratner P, Bernstein D. Desloratadine Urticaria Study Group. Efficacy and safety of desloratadine 5 mg once daily in the treatment of chronic idiopathic urticaria: a double-blind, randomised, placebo-controlled trial. J Am Acad Dermatol. 2003;48:535–541 [DOI] [PubMed] [Google Scholar]
- 26.Simons FER, Prenner BM, Finn A., Jr Desloratadine Study Group Efficacy and safety of desloratadine in the treatment of perennial allergic rhinitis. J Allergy Clin Immunol. 2003;111:617–622 [DOI] [PubMed] [Google Scholar]
- 27.Ring J, Hein R, Gauger A, Bronsky E, Miller B, Desloratadine Study Group Once-daily desloratadine improves the signs and symptoms of chronic idiopathic urticaria: a randomised, double-blind, placebo-controlled study. Int J Dermatol. 2001;40:72–76 [DOI] [PubMed] [Google Scholar]
- 28.Aerius Summary of Product Characteristics. Brussels, Belgium: SP Europe; 2007 [Google Scholar]
- 29.Clarinex® (desloratadine) product information. Kenilworth, NJ: Schering-Plough; 2007 [Google Scholar]
- 30.Bakker RA, Schoonus SB, Smit MJ, Timmerman H, Leurs R. Histamine H(1)-receptor activation of nuclear factor-kappa B: roles for G beta gamma- and G alpha(q/11)-subunits in constitutive and agonist-mediated signaling. Mol Pharmacol. 2001;60:1133–1142 [DOI] [PubMed] [Google Scholar]
- 31.Greasley PJ, Clapham JC. Inverse agonism or neutral antagonism at G-protein coupled receptors: a medicinal chemistry challenge worth pursuing? Eur J Pharmacol. 2006;553:1–9 [DOI] [PubMed] [Google Scholar]
- 32.National Center for Biomedical Information [Entrez Gene Website.] NFKB1: nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 [[Homo sapiens]]. Available at: http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=4790&ordinalpos=6&itool=EntrezSystem2.PEntrez.Gene.Gene_ResultsPanel.Gene_RVDocSum Accessed July 11, 2010.
- 33.Costa T, Cotecchia S. Historical review: negative efficacy and the constitutive activity of G-protein-coupled receptors. Trends Pharmacol Sci. 2005;26:618–624 [DOI] [PubMed] [Google Scholar]
- 34.de Ligt RA, Kourounakis AP, IJzerman AP. Inverse agonism at G protein-coupled receptors: (patho)physiological relevance and implications for drug discovery. Br J Pharmacol. 2000;130:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakker RA, Wieland K, Timmerman H, Leurs R. Constitutive activity of the histamine H(1) receptor reveals inverse agonism of histamine H1 receptor antagonists. Eur J Pharmacol. 2000;387:R5–R7 [DOI] [PubMed] [Google Scholar]
- 36.Anthes JC, Gilchrest H, Richard C Eckel S, Hesk D, et al. Biochemical characterization of desloratadine, a potent antagonist of the human histamine H1 receptor. Eur J Pharmacol. 2002;449:229–237 [DOI] [PubMed] [Google Scholar]
- 37.Wu R-L, Anthes JC, Kreutner W, Harris AG, West RE., Jr Desloratadine inhibits constitutive and histamine-stimulated nuclear factor-κB activity consistent with inverse agonism at the histamine H1 receptor. Int Arch Allergy Immunol. 2004;135:313–318 [DOI] [PubMed] [Google Scholar]
- 38.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–174 [DOI] [PubMed] [Google Scholar]
- 39.Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111(Suppl):S460–S475 [DOI] [PubMed] [Google Scholar]
- 40.Lebel B, Bousquet J, Czarlewski W, Campbell AM. Loratadine (L) reduces RANTES release by an epithelial cell line. J Allergy Clin Immunol. 1997;99:S444 Abstract 1802. [Google Scholar]
- 41.Kowalski ML, Lewandowska A, Wozniak J, Makowska J, Jankowski A, DuBuske L. Inhibition of nasal polyp mast cell and eosinophil activation by desloratadine. Allergy. 2005;60:80–85 [DOI] [PubMed] [Google Scholar]
- 42.Di Sciascio MB, Vianale G, Verna N, Petrarca C, Perrone A, et al. Eosinophil recruiting chemokines are down-regulated in peripheral blood mononuclear cells of allergic patients treated with deflazacort or desloratadine. Int J Immunopathol Pharmacol. 2007;20:745–751 [DOI] [PubMed] [Google Scholar]
- 43.Cyr MM, Hayes LM, Crawford L, Baatjes AJ, Keith PK, Denburg JA. The effect of desloratadine on eosinophil/basophil progenitors and other inflammatory markers in seasonal allergic rhinitis: a placebo-controlled randomized study. Int Arch Allergy Immunol. 2005;138:209–216 [DOI] [PubMed] [Google Scholar]
- 44.Mullol J, Roca-Ferrer J, Alobid I, Pujols L, Valero A, et al. Effect of desloratadine on epithelial cell granulocyte-macrophage colony-stimulating factor secretion and eosinophil survival. Clin Exp Allergy. 2006;36:52–58 [DOI] [PubMed] [Google Scholar]
- 45.Wang YH, Taché Y, Harris AG, Kreutner W, Daly AF, Wei JY. Desloratadine prevents compound 48/80-induced mast cell degranulation: visualization using a vital fluorescent dye technique. Allergy. 2005;60:117–124 [DOI] [PubMed] [Google Scholar]
- 46.Genovese A, Patella V, De Crescenzo G, De Paulis A, Spadaro G, Marone G. Loratadine and desethoxylcarbonyl-loratadine inhibit the immunological release of mediators from human FcεRI+ cells. Clin Exp Allergy. 1997;27:559–567 [DOI] [PubMed] [Google Scholar]
- 47.Lippert U, Krüger-Krasagakes S, Möller A, Kiessling U, Czarnetzki BM. Pharmacological modulation of IL-6 and IL-8 secretion by the H1-antagonist decarboethoxy-loratadine and dexamethasone by human mast and basophilic cell lines. Exp Dermatol. 1995;4(part 2):272–276 [DOI] [PubMed] [Google Scholar]
- 48.Weller K, Maurer M. Desloratadine inhibits human skin mast cell activation and histamine release. J Invest Dermatol. 2009;129:2723–2726 [DOI] [PubMed] [Google Scholar]
- 49.Tworek D, Bochenska-Marciniak M, Kupczyk M, Górski P, Kuna P. The effect of 4 weeks treatment with desloratadine (5 mg daily) on levels of interleukin (IL)-4, IL-10, IL-18 and TGF beta in patients suffering from seasonal allergic rhinitis. Pulm Pharmacol Ther. 2006;20:244–249 [DOI] [PubMed] [Google Scholar]
- 50.Schroeder JT, Schleimer RP, Lichtenstein LM, Kreutner W. Inhibition of cytokine generation and mediator release by human basophils treated with desloratadine. Clin Exp Allergy. 2001;31:1369–1377 [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Leung PC, Woo KS, Chen GG, Wong YO, Liu SX, van Hasselt CA. Inhibitory effects of budesonide, desloratadine and dexamethasone on cytokine release from human mast cell line (HMC-1). Inflamm Res. 2004;53:664–669 [DOI] [PubMed] [Google Scholar]
- 52.Molet S, Gosset P, Lassalle P, Czarlewski W, Tonnel A-B. Inhibitory activity of loratadine and descarboxyethoxyloratadine on histamine-induced activation of endothelial cells. Clin Exp Allergy. 1997;27:1167–1174 [PubMed] [Google Scholar]
- 53.Vignola AM, Crampette L, Mondain M, Sauvère G, Czarlewski W, Bousquet J, Campbell AM. Inhibitory activity of loratadine and descarboethoxyloratadine on expression of ICAM-1 and HLA-DR by nasal epithelial cells. Allergy. 1995;50:200–203 [DOI] [PubMed] [Google Scholar]
- 54.Traidl-Hoffmann C, Münster I, Ring J, Behrendt H. Impact of desloratadine and loratadine on the crosstalk between human keratinocytes and leukocytes: implications for anti-inflammatory activity of antihistamines. Int Arch Allergy Immunol. 2006;140:315–320 [DOI] [PubMed] [Google Scholar]
- 55.DuBuske L. Desloratadine for chronic idiopathic urticaria: a review of clinical efficacy. Am J Clin Dermatol. 2007;8:271–283 [DOI] [PubMed] [Google Scholar]
- 56.DuBuske LM. Review of desloratadine for the treatment of allergic rhinitis, chronic idiopathic urticaria and allergic inflammatory disorders. Expert Opin Pharmacother. 2005;6:2511–2523 [DOI] [PubMed] [Google Scholar]
- 57.Agrawal DK. Pharmacology and clinical efficacy of desloratadine as an anti-allergic and anti-inflammatory drug. Expert Opin Investig Drugs. 2001;10:547–560 [DOI] [PubMed] [Google Scholar]
- 58.Ciprandi G, Cirillo I. Rupatadine improves nasal symptoms, airflow and inflammation in patients with persistent allergic rhinitis: a pilot study. J Biol Regul Homeost Agents. 2010;24:177–183 [PubMed] [Google Scholar]
- 59.Ciprandi G, Cirillo I, Mora F, La Rosa M. Ebastine improves nasal symptoms and airflow and affects response to decongestion test in patients with persistent allergic rhinitis: a pilot study. Allergy Asthma Proc. 2007;28:578–581 [DOI] [PubMed] [Google Scholar]
- 60.Ciprandi G, Cirillo I, Vizzaccaro A, Civardi E, Barberi S, et al. Desloratadine and levocetirizine improve nasal symptoms, airflow, and allergic inflammation in patients with perennial allergic rhinitis: a pilot study. Int Immunopharmacol. 2005;5:1800–1808 [DOI] [PubMed] [Google Scholar]
- 61.Ciprandi G, Cirillo I, Vizzaccaro A, Tosca MA. Levocetirizine improves nasal symptoms and airflow in patients with persistent allergic rhinitis: a pilot study. Eur Ann Allergy Clin Immunol. 2005;37:25–29 [PubMed] [Google Scholar]
- 62.Gillard M, Strolin Benedetti M, Chatelain P, Baltes E. Histamine H1 receptor occupancy and pharmacodynamics of second generation H1-antihistamines. Inflamm Res. 2005;54:367–369 [DOI] [PubMed] [Google Scholar]


