Figure 2.
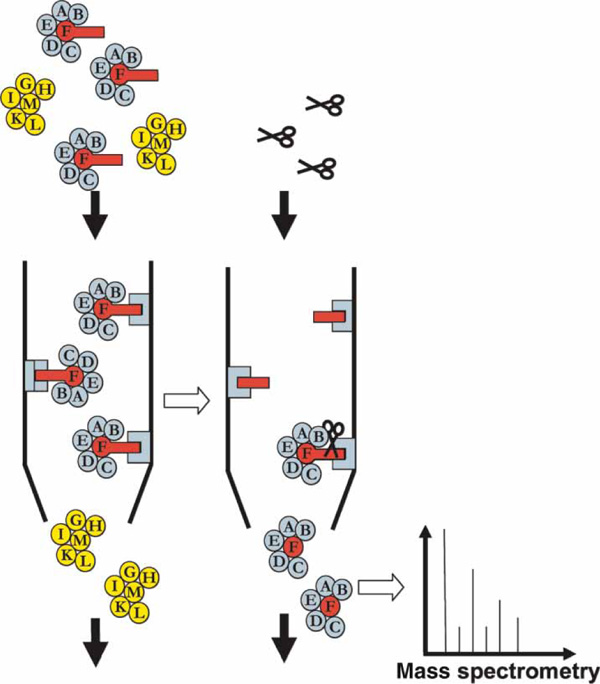
An affinity purification experiment followed by mass spectrometry. The protein of interest, F (red circle), is fused to a protein fragment -- the 'tag' (red rectangle). The tag allows this protein to be purified biochemically. Proteins binding to the tagged protein (blue) are co-purified, whereas proteins not binding to protein F (yellow) are discarded. The purified proteins can be released using enzymatic cleavage (scissors) or other methods, depending on the nature of the tag. These proteins are then identified by mass spectrometry.
