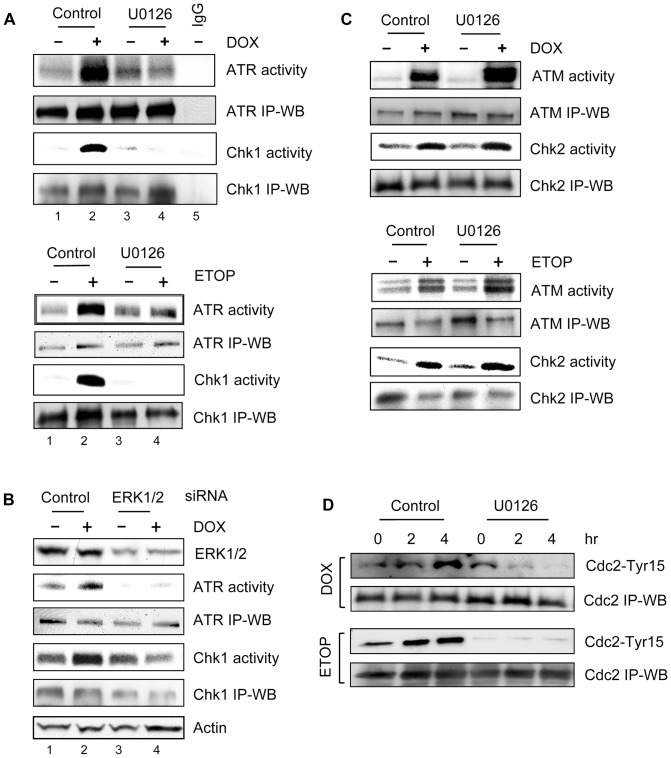
Figure 6. Effect of ERK1/2 inhibition on topo II poison-induced ATR and ATM signaling activation.
(A) MCF-7 cells were treated for 2 hr with 1 µM DOX (upper panel) or 10 µM ETOP (lower panel) in the presence or absence of 50 µM U0126. The cells were washed and incubated in growth medium for additional 2 hr with the presence or absence of U0126. ATR and Chk1 kinase were respectively immunoprecipitated from the resulting cell lysates and assayed for kinase activity. ATR and Chk1 levels in immunoprecipitates were determined by immunoblotting (ATR IP-WB and Chk1 IP-WB). IgG, as a negative control, kinase assay was carried out using immunoprecipitates obtained by incubating control untreated cell lysate with non-immunized IgG. (B) Cells transfected with ERK1/2 specific or control siRNA were incubated for 2 days and treated with or without 0.5 µM DOX, as described above. ATR and Chk1 were respectively immunoprecipitated from the cell lysates and examined for kinase activity (ATR activity and Chk1 activity). ATR and Chk1 protein levels in immunoprecipitates were determined by immunoblotting (ATR IP-WB and Chk1 IP-WB). Levels of ERK1/2 and Actin in cell lysates were analyzed by immunoblotting (ERK1/2 and Actin). (C) Cells were treated as described in (A). ATM and Chk2 were immunoprecipitated from the cell lysates and assayed for kinase activity (ATM activity and Chk2 activity). ATM and Chk2 levels in immunoprecipitates were determined by immunoblotting (ATM IP-WB and Chk2 IP-WB). (D) MCF-7 cells were treated as described in (A) and incubated for the times indicated. Cdc2 was immunoprecipitated from cell lysate and analyzed for Cdc2-Tyr15 phosphorylation by immunoblotting (Cdc2-Tyr15). Cdc2 in the immunoprecipitates was quantified by immunoblotting (Cdc-2 IP-WB).