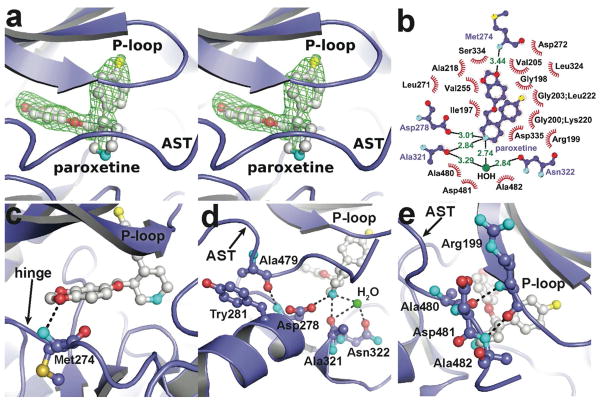
Figure 3. Atomic structure of the GRK2·paroxetine-Gβγ complex.
(a) Stereo-view of paroxetine bound in the active site of GRK2. Electron density from an m|Fo| − D|Fc| omit map contoured at 3 σ is shown as a green cage. (b) Schematic of GRK2 interactions with paroxetine. Residues that form hydrogen-bonds (dashed lines) with paroxetine are shown in ball-and-stick representation with the interatomic distances shown in Å. Residues forming van der Waal interactions with paroxetine are shown as labeled arcs with radial spokes that point toward the ligand atoms they interact with. (c and d) Interactions of paroxetine with residues forming the adenine and ribose subsites, respectively. (e) Paroxetine binding stabilizes the AST region of GRK2, which contacts the inhibitor and forms novel interactions with the phosphate-binding loop (P-loop). Carbons for GRK2 and paroxetine are shown in slate and white, respectively. Nitrogens are colored cyan, oxygens red, and sulfur and fluorine yellow.