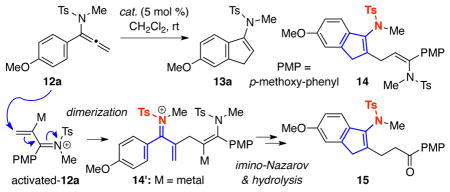
Table 1.
Screening for a Suitable Metal Catalyst.
| |||||
|---|---|---|---|---|---|
| entrya | catalyst | time (h) | conversion (%)b | 13a (%)c | 14 + 15d (%)c |
| 1 | AuCl3 | 30 | >95 | – | 55 |
| 2 | AuCl3/AgSbF6 | 1 | >95 | – | 45 |
| 3 | AuCl3/AgSbF6e | 22 | 48 | – | 36 |
| 4 | AuCl3/AgSbF6f | 40 | 55g | – | 36 |
| 5 | AuCl/AgSbF6 | 5 | >95 | – | 48 |
| 6 | AuCl(PPh3)/AgSbF6 | 60 | 75g | 50h | 24 |
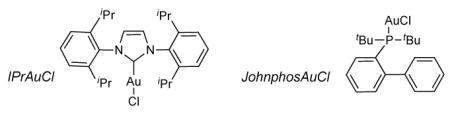
| 7 | JohnphosAuCl/AgSbF6 | 5 | >95 | 91 | – |
| 8 | IPrAuCl/AgSbF6 | 1 | >95 | 97 | – |
| 9 | IPrAuCl | 21 | <5 | – | – |
| 10 | AgSbF6i | 20 | 64 | – | 24 |
| 11 | PtCl2 | 24 | <5 | – | – |
| 12 | PtCl4i | 12 | >95 | – | 82j |
Unless noted, all reactions were carried out at 0.1 mmol scale in 2 mL of CH2Cl2 (concn = 0.05 M) at room temperature with the addition of 5 mol % catalyst.
Conversion determined by 1H NMR.
Isolated yields.
14 and 15 could not be separated by column chromatography.
20 mL CH2Cl2 was used (concn = 0.005 M).
35 mg 4 Å MS was added.
Based on recovered 12a.
Inseparable mixture with 12a.
10 mol % of catalyst was used.
An inseparable mixture of 14, 15 and unidentified products.