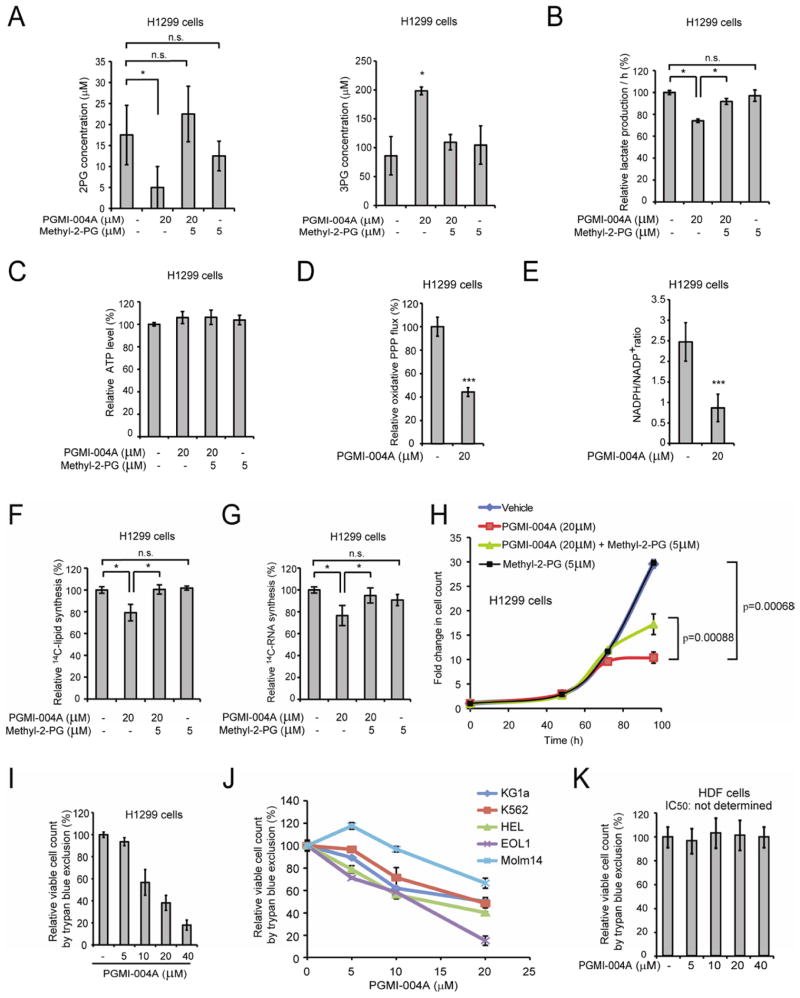
Figure 7. Inhibition of PGAM1 by PGMI-004A reveals that PGAM1 enzyme activity is important for regulation of 3-PG and 2-PG levels and coordination of glycolysis and biosynthesis to promote cancer cell proliferation.
(A) 2-PG (left) and 3-PG (right) levels in H1299 cells treated with or without PGMI-004A were determined in the presence and absence of methyl-2-PG.
(B–C) Lactate production (B) and intracellular ATP levels (C) in H1299 cells treated with or without PGMI-004A were determined in the presence and absence of methyl-2-PG.
(D–E) H1299 cells treated with or without PGMI-004A were tested for oxidative PPP flux (D) and NADPH/NADP+ ratio (E).
(F–H) H1299 cells treated with or without PGMI-004A were tested for biosynthesis of lipids (F) and RNA (G), as well as cell proliferation (H) in the presence and absence of methyl-2-PG.
(I–K) Cell viability of H1299 cells (I), diverse human leukemia cells (J) and control human dermal fibroblasts (HDF) cells (K) in the presence of increasing concentrations of PGMI-004A. Cell viability was determined by trypan blue exclusion.
The error bars represent mean values +/− SD from three replicates of each sample (*: 0.01<p<0.05; ***: p<0.001; n.s.: not significant).
See also Figure S5.