Abstract
Post-proline cleaving peptidases are promising therapeutic targets for neurodegenerative diseases, psychiatric conditions, metabolic disorders, and many cancers. Prolyl oligopeptidase (POP; E.C. 3.4.21.26) and fibroblast activation protein α (FAP; E.C. 3.4.24.B28) are two post-proline cleaving endopeptidases with very similar substrate specificities. Both enzymes are implicated in numerous human diseases, but their study is impeded by the lack of specific substrate probes. We interrogated a combinatorial library of proteolytic substrates and identified novel and selective substrates of POP and FAP. These new sequences will be useful as probes for fundamental biochemical study, scaffolds for inhibitor design, and triggers for controlled drug delivery.
Keywords: Fibroblast activation protein α, prolyl oligopeptidase, prolyl endopeptidase, Alzheimer’s disease, Parkinson’s disease, depression, cognition, cancer, tumor stromal fibroblasts, protease substrate profiling, internally quenched fluorogenic probes
Introduction
A major challenge in the development of pharmacologically viable protease inhibitors is achieving sufficient selectivity to avoid undesirable off-target effects [1]. Highly specific substrates and probes are also needed to enable fundamental studies of the roles of proteases in health and disease. We have undertaken a biochemical study of prolyl oligopeptidase and fibroblast activation protein α, two post-proline cleaving endopeptidases with very similar substrate specificities that are proposed therapeutic targets for major human diseases.
Prolyl oligopeptidase (POP; E.C. 3.4.21.26) is an 80 kDa soluble endopeptidase that cleaves peptides less than 30 amino acids in length C-terminal to proline residues, including many neuroactive peptides [2]. POP is expressed in virtually all tissues, though particularly high levels are found in the brain and central nervous system. POP has traditionally been regarded as a cytosolic enzyme, but activity has also been reported in extracellular compartments, including cerebrospinal fluid, serum, and lung fluid [2–4]. Levels of POP activity are altered in many neurodegenerative conditions and psychiatric disorders, leading to interest in POP as a therapeutic target for these diseases. POP can be differentiated from FAP in vitro via its selective inhibition by Z-Pro-Prolinal (ZPP) [5]. Although POP inhibitors are undergoing preclinical and clinical evaluation, their development has been impeded because the roles of POP in disease pathogenesis have not been fully elucidated [6].
Fibroblast activation protein α (FAP, also called seprase; E.C. 3.4.24.B28) is a type II integral membrane serine protease [7]. A truncated soluble form of FAP, called antiplasmin-cleaving enzyme (APCE), has also been reported [8]. FAP is closely related to dipeptidyl peptidase IV (DPPIV) and exhibits post-proline cleaving dipeptidyl peptidase activity similar to DPPIV, but unlike DPPIV it also exhibits endopeptidase activity toward gelatin and α2-antiplasmin [9]. FAP specificity is most commonly distinguished from POP through its requirement for Gly at the P2 position [10]. In contrast to POP, FAP is not expressed in normal adult tissues, but is highly expressed on stromal fibroblasts in virtually all epithelial carcinomas and on tumor cells of some sarcomas [11]. FAP has been implicated in tumorigenesis in animals, and FAP activity has been associated with metastasis and poor prognosis in some human cancers [12]. Collectively, these studies have aroused interest in FAP as a target for cancer therapy, but validation efforts have been hampered by the lack of highly selective inhibitors.
Recent studies have cast doubt on the traditional delineation between the proteolytic activities of POP and FAP. Z-Gly-Pro-AMC and other substrate-based probes that have been used to assay POP activity in cells, tissues, and body fluids are now known to be cleaved by FAP as well, potentially confounding the results of those studies [2,8]. In addition, FAP (or APCE, its soluble form) has been shown to contribute a portion of post-proline cleaving endopeptidase activity in serum and other tissues [13]. POP activity affects cognitive function and may be altered in neurodegenerative diseases and psychiatric disorders, but studies conflict as to whether POP activity is increased or decreased, and in most cases the physiologic role of POP is unclear [6]. Furthermore, FAP has recently been reported to cleave some neuroactive POP substrates [14]. In addition, both FAP and POP play roles in inflammation and immunomodulation, though they are incompletely understood [4,15]. Thus, there is a clear need to clarify the substrate specificities of these two enzymes to facilitate the design of selective inhibitors and to enable fundamental biochemical studies of their roles in disease processes. To address this need, we employed a combinatorial library of internally quenched fluorogenic probes to comparatively profile the substrate specificities of POP and FAP.
Materials and methods
Materials
Human recombinant POP, human recombinant FAP, and POP inhibitor ZPP were obtained from R&D Systems (Minneapolis, MN). Cleavage of the generic probe Z-Gly-Pro-AMC by both enzymes was consistent and reproducible across enzyme lots (Figure S1 and S2). POP and FAP were supplied at 0.5 mg/mL, diluted to 50 μg/mL in their respective storage buffers (as recommended by the manufacturer), and stored at −80 °C until use. All other reagents and materials were obtained from Sigma-Aldrich (St. Louis, MO) or VWR (Radnor, PA).
Substrate specificity profiling
Analysis of POP and FAP substrate specificities was performed using a library of 3375 internally quenched fluorogenic probes (IQFPs) (Mimotopes, Clayton, Victoria, Australia). These probes remain optically silent in the uncleaved state, but upon cleavage they emit a fluorescent signal with intensity proportional to the extent of cleavage. Peptides in this library contain the sequence MCA-Gly-Gly-Gly-Xaa-Yaa-Zaa-Gly-Gly-DPA-Lys-Lys, in which Xaa, Yaa, and Zaa correspond to variable residues comprising equimolar mixtures of Ala/Val, Asp/Glu, Phe/Tyr, Ile/Leu, Lys/Arg, Asn/Gln, Ser/Thr, or Pro (Figure 1A). Thus, each well of the library contains an equimolar mixture of up to eight individual peptides. This library has been validated previously through substrate specificity profiling of recombinant proteases from each of the major protease classes [16].
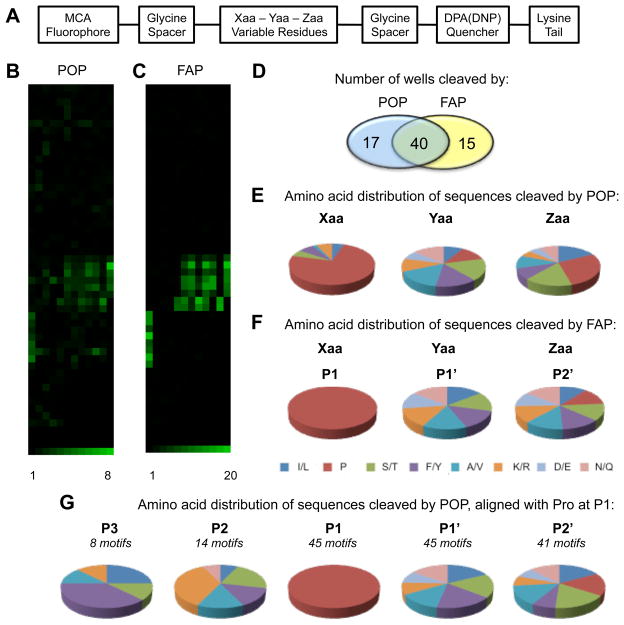
Figure 1. Substrate specificities of POP and FAP.
(A) Schematic illustration of a representative internally quenched fluorogenic probe. (B-C) Graphical heatmap representations of the substrate specificities of POP (B) and FAP (C). Colored squares represent individual wells of stacked 96 well microplates [3]. The numbers at the bottom of the heat map corresponds to the fluorescence fold change values (upper and lower limit) used in generating the heat maps. The fold change values were calculated as described in the materials and methods section (D) Venn diagram indicating the numbers of selective and promiscuous IQFP motifs detected. (E–F) Amino acid distribution of all IQFP sequences cleaved by POP (E) and FAP (F). Individual motifs are listed in Tables 1 and S1 (G) Amino acid distribution of IQFP sequences cleaved by POP aligned with Pro fixed at P1. The % amino acid distribution values for each pie chart are presented in Tables S3 and S4.
IQFP library screens were performed as described [3] with the following modifications. Before starting the assay, aliquots of frozen POP and FAP were thawed on ice and diluted to 10 μg/mL in the assay buffers recommended by the manufacturer (POP −25 mM Tris, 250 mM NaCl, 2.5 mM DTT, pH 7.5; FAP −50 mM Tris, 1 M NaCl, 1 mg/mL BSA, pH 7.5). The reaction was initiated at t = 0 min by the addition of IQFP. In each well, the final IQFP concentration was 62.5 μM and the final enzyme concentration was 1 μg/mL. Endpoint fluorescence intensity fold change after 6 h at room temperature was calculated as Ffinal/Finitial. No fluorescence enhancement was observed in wells lacking enzyme (data not shown). Library wells were considered to be cleaved if an endpoint fluorescence intensity fold change value greater than 2 was observed.
Deconvolution and inhibition assays
The individual sequences derived from each selected IQFP motif were assayed individually as described [3] with the following modifications. IQFPs were custom synthesized, confirmed by mass spectrometry, and provided as lyophilized powders (Mimotopes). IQFP stock solutions were prepared in dimethylsulfoxide (DMSO) and diluted to 1 mM in assay buffer (assay buffers as described above). Enzyme stock solutions were diluted to 10 μg/mL in assay buffer immediately before use. In each assay well, the final IQFP concentration was 25 μM and the final enzyme concentration was 1 μg/mL.
Where indicated, substrate cleavage sites were confirmed by identification of N-terminal and C-terminal substrate fragments by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry using an ABI 4800 MALDI-TOF/TOF mass spectrometer (Applied Biosystems, Carlsbad, CA) as described [3]. To determine the effect of POP inhibitor ZPP on cleavage of selected IQFPs, experiments were conducted as described except enzyme solutions (1 μg/mL) were incubated with 5 μM ZPP for 30 min at room temperature prior to the start of the experiment. Experiments using physiologically derived substrates were performed as describe above. Sequences were synthesized as indicated in Table 1, with MCA-Gly-Gly appended to the N-terminus and Gly-Gly-DPA-Lys-Lys appended to the C-terminus.
Table 1.
Substrate sequences cleaved selectively by POP or by FAP.a
| Cleaved only by POP | Cleaved only by FAP | ||||||
|---|---|---|---|---|---|---|---|
| Xaa | Yaa | Zaa | Fold Change | Xaa | Yaa | Zaa | Fold Change |
| P | D/E | I/L | 2.41 | P | A/V | D/E | 3.45 |
| P | P | F/Y | 2.30 | P | A/V | K/R | 10.61 |
| F/Y | P | I/L | 2.13 | P | D/E | A/V | 4.42 |
| I/L | P | I/L | 2.67 | P | D/E | K/R | 4.72 |
| K/R | P | I/L | 2.30 | P | D/E | N/Q | 2.38 |
| K/R | P | F/Y | 2.33 | P | F/Y | F/Y | 11.45 |
| K/R | P | S/T | 2.04 | P | I/L | D/E | 4.44 |
| S/T | P | S/T | 2.34 | P | I/L | F/Y | 5.38 |
| A/V | K/R | P | 2.11 | P | I/L | K/R | 9.21 |
| F/Y | A/V | P | 4.19 | P | K/R | D/E | 6.46 |
| F/Y | K/R | P | 2.31 | P | K/R | F/Y | 8.17 |
| F/Y | S/T | P | 2.39 | P | K/R | K/R | 10.62 |
| I/L | N/Q | P | 2.39 | P | K/R | N/Q | 16.64 |
| I/L | S/T | P | 3.55 | P | N/Q | F/Y | 9.59 |
| K/R | A/V | P | 2.10 | ||||
| S/T | F/Y | P | 2.03 | ||||
Selective IQFP motifs were identified by screening a combinatorial substrate library. For each sequence, the endpoint fluorescence fold change value is shown. IQFP wells were considered to be cleaved if fold change values greater than 2 were observed.
Statistical analysis
Statistical significance was assessed by analysis of variance and two-tailed Student’s t-test. Equality of variance was determined by F-test. Differences were considered significant if they exhibited p values < 0.05 in Student’s t-test. Data analyses were performed using Microsoft Excel and AnalystSoft StatPlus. All measurements were obtained in duplicate. All data presented are representative of at least two independent experiments performed with separate enzyme preparations.
Results and discussion
Substrate specificity profiling
Highly specific substrate probes are needed to enable biochemical studies of prolyl oligopeptidase and fibroblast activation protein α and to facilitate discovery of selective inhibitors for these potential therapeutic targets. We have performed the first unbiased direct comparison of the proteolytic activities of POP and FAP, which provided new information about the substrate specificities of both enzymes and identified selective probes (Figure 1). While 40 IQFP motifs were cleaved by both enzymes (Table S1), a considerable number of wells were cleaved by only one (17 for POP and 15 for FAP) (Table 1). Both enzymes exclusively cleaved substrates containing Pro.
An analysis of the amino acid distribution of POP substrates revealed that Pro is strongly preferred, although not absolutely required, at the Xaa position (74% of all POP substrates; Figure 1E). With the exception of Asn/Gln, all other residues were represented in Xaa at least once. A substantial fraction of POP substrates contained Pro at the Yaa and Zaa positions as well (12% and 28%, respectively). POP was also found to cleave one motif in which Pro was found at both the Xaa and Yaa positions (Pro-Pro-Phe/Tyr), albeit weakly. In all substrates cleaved by POP, charged residues were strongly disfavored at the position following Pro; Arg/Lys represented 11% of Yaa and 5% of Zaa, while Asp/Glu represented 7% of Yaa and 5% of Zaa. When POP substrates were aligned with Pro fixed at the P1 position, the bias against charged residues mapped to the P1′ and P2′ positions (Figure 1G). In contrast, cationic residues were prominent in the P2 position, consistent with a study of POP from porcine kidney in which cationic residues were preferred over anionic residues at P2 [17].
Surprisingly few studies of POP substrate specificity based on positional scanning libraries have been published. Gorrao and coworkers interrogated POP cleavage of a positional scanning library based on bradykinin and observed a preference for Arg/Asn/Ile/Leu at the P2 and P3 positions, while P1′ and P2′ were promiscuous [18]. A comparative study of three bacterial POPs revealed a preference for Gln or Tyr at P1′ [19]. Another bacterial POP exhibited a preference for Phe/Leu at P1′ and little preference at P2′ [20], although Asp/Lys were the least favored residues at this position, consistent with our findings. However, extensibility of these observations from bacterial to human POP is unknown.
In contrast to POP, FAP exhibited an absolute requirement for Pro in the Xaa position, consistent with the well-known Gly-Pro FAP cleavage site [10,21,22]. Also unlike POP, FAP did not exhibit a clear preference for any residue at P1′ or P2′. Among FAP substrates, no residue represented less than 13% and 11% of P1′ and P2′, respectively (Figure 1F). The lone exception was Pro, which was not found at P1′ in any FAP substrates. These data deviate somewhat from previous studies. Positional scanning probe libraries based on the α2-antiplasmin cleavage site indicated preferences for Ala/Tyr/Ser/Asn at P1′ and Phe/Tyr at P2′ [10,22]. Mass spectrometry-based analysis of collagen I-derived gelatin digested by FAP also suggested a preference for Ala at P1′ [21]. These differences may be highly dependent on the context of the overall peptide sequence; while previous positional scanning libraries were designed from physiological substrates [10,22], our study is the first to interrogate an unbiased combinatorial substrate library.
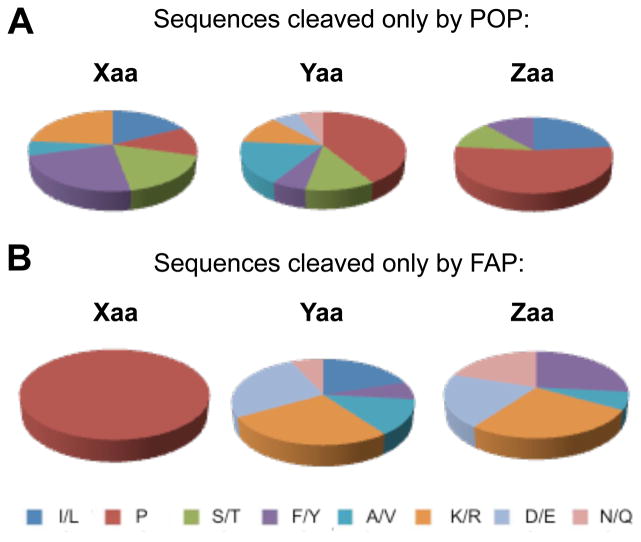
We also analyzed the substrate sequences cleaved exclusively by only one of the two enzymes, revealing several key observations (Figure 2 and Table 1). First, FAP-specific IQFPs contain Pro exclusively at the Xaa position, whereas all but one of the POP-specific substrates contained Pro at either Yaa or Zaa. Second, charged residues (Arg/Lys and Asp/Glu) are disfavored at P1′ and P2′ in POP-specific substrates, but are preferred in the same positions in FAP-specific substrates (54% and 47% of P1′ and P2′, respectively). Third, Ser/Thr residues are completely absent from FAP-specific sequences. These observations could serve as the basis for future design of highly selective probes and inhibitors. These guidelines can also be used in the design of FAP-activated prodrugs, which have been recently sought as a means of targeting the microenvironment of epithelial tumors but are vulnerable to undesired cleavage by POP [23].
Figure 2. Amino acid distribution of selective probe sequences.
In POP-specific sequences (A), Pro was found at all three variable positions and charged residues were disfavored at P1′ and P2′. In FAP-specific sequences (B), Pro was found exclusively at Xaa and charged residues were heavily favored at P1′ and P2′. The % amino acid distribution values for each pie chart are presented in Table S5.
Deconvolution of selected IQFP motifs
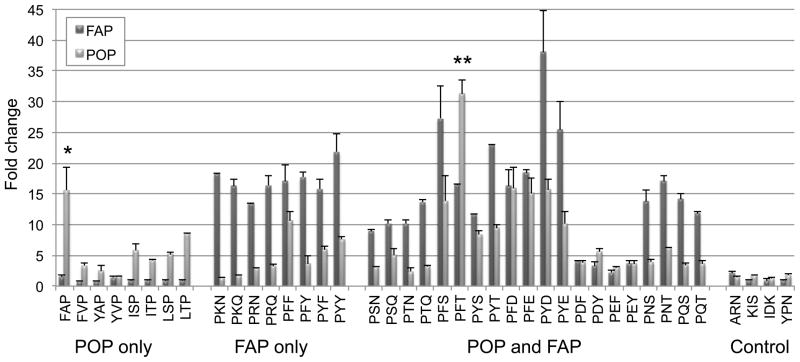
To further characterize the substrate specificities of POP and FAP, constituent sequences comprising two POP-specific motifs, two FAP-specific motifs, and four dual-specificity motifs were individually synthesized and confirmed by mass spectrometry. Fine amino acid substrate specificities of these motifs were determined by quantifying endpoint fluorescence fold change following incubation of either POP or FAP with each individual substrate (Figure 3). Exclusivity of both POP-specific motifs was confirmed upon deconvolution. Among the constituent sequences of the motif Phe/Tyr-Ala/Val-Pro, the substrate Phe-Ala-Pro was strongly preferred. In contrast, the 4 sequences comprising the motif Ile/Leu-Ser/Thr-Pro were all cleaved to a similar extent. Among the constituent sequences of the two motifs believed to be FAP-specific (Pro-Arg/Lys-Asn/Gln and Pro-Phe/Tyr-Phe/Tyr), FAP did not exhibit any clear preferences. Upon deconvolution, one of these two motifs (Pro-Phe/Tyr-Phe/Tyr) was also cleaved by POP, underscoring the need for secondary confirmation of substrate motifs derived from combinatorial library screens.
Figure 3. Deconvolution of IQFP substrates.
Extent of cleavage is expressed as endpoint fluorescence fold change at 6 h. Dark bars denote cleavage by FAP, while light bars denote cleavage by POP. Error bars represent standard deviations. * p < 0.05 versus FVP and YAP for cleavage by POP. ** p < 0.05 versus PFS, PYS, and PYT for cleavage by POP.
Of the dual-specificity motifs that were selected for deconvolution, all individual sequences were cleaved by both enzymes. Notable substrate specificity preferences among these sequences include Pro-Phe-Thr, which was strongly cleaved by POP, and Pro-Tyr-Asp, which was strongly cleaved by FAP. One noteworthy observation was the inability to exchange Asp/Glu and Phe/Tyr at the P1′ and P2′ positions; Pro-Phe/Tyr-Asp/Glu sequences were extensively cleaved by both POP and FAP, but neither enzyme exhibited substantial cleavage of Pro-Asp/Glu-Phe-Tyr. In addition, POP exhibited only modest cleavage of the motifs Pro-Ser/Thr-Asn/Gln and Pro-Asn/Gln-Ser/Thr. Post-proline cleavage of deconvoluted substrates was also confirmed by MALDI mass spectrometry (Table S2). A subset of these sequences was used to assay the inhibitory effects of ZPP against both FAP and POP (Figure S3). As expected, cleavage of all substrates by POP was completely inhibited by 5 μM ZPP (>93% inhibition for all substrates tested), whereas FAP activity was unaffected.
Cleavage of physiologically derived substrates
We sought to determine if the overlap in POP and FAP substrate specificities we detected in this 3-mer IQFP library may also extend to physiologic substrates. Although FAP has recently been reported to exhibit dipeptidyl peptidase activity toward several neuropeptides, including POP substrates [14], there has been little investigation of the potential overlap between the activities of these two enzymes. This is particularly important in elucidating the physiologic roles of POP, which is widely expressed and exhibits less stringent substrate specificity than FAP. Thus, we designed and synthesized IQFPs based on two POP substrates (bradykinin and substance P) and two FAP substrates (gelatin and α-antiplasmin) that contained the same fluorophore-quencher probe architecture as the peptides in our IQFP library (Table 2) [10,18,21,24].
Table 2.
Summary of physiologically relevant peptides.a
| Peptide | Source | Proteaseb | Reference | POP cleavage site(s)c | FAP cleavage site(s) c |
|---|---|---|---|---|---|
| GFSPFRQED | Bradykinin | POP | [18] | GFSP‡ FRQED | GFSPFRQED‡ |
| RPKPQQFFGLM | Substance P | POP | [24] | RP‡ KP‡ QQFFGLM | RPKP‡ QQ‡ FF‡ GLM |
| GASGPAGPA | Gelatin | FAP | [21] | None found | GASGP‡ AGPA |
| GEPGPPGPA | Gelatin | FAP | [21] | GEP‡ GPPGP‡ A | GEP‡ GPPGP‡ A |
| GTSGPNQEQE | α-antiplasmin | FAP | [10] | GTSGP‡ NQEQE | GTSGP‡ NQEQE |
| GTAGPNQEQE | α-antiplasmin | FAP | [10] | GTAGP‡ NQEQE | GTAGP‡ NQEQE |
Physiologically derived substrates were adapted from the indicated literature references and modified by addition of the MCA-DPA fluorophore-quencher pair.
indicates a cleavage site.
The canonical protease according to literature is indicated.
As determined by MALDI mass spectrometry.
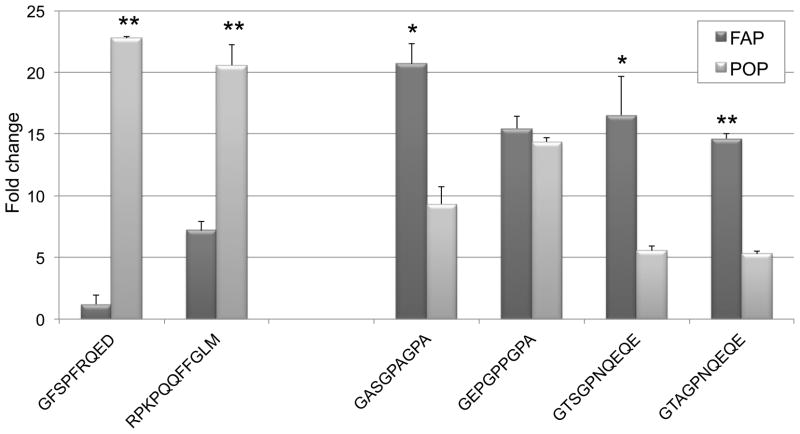
We confirmed that all substrates were cleaved by their cognate enzymes as expected (Figure 4). However, we also detected considerable promiscuous cleavage. A POP substrate derived from substance P was cleaved by FAP, and all four FAP substrates (derived from gelatin and α-antiplasmin) were cleaved by POP. MALDI mass spectrometry analysis confirmed post-proline cleavage sites (Table 2). Strikingly, the substance P-derived sequence Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met, which does not contain Gly-Pro, was reproducibly cleaved by FAP. This may suggest that the requirement for a Gly-Pro motif is not ironclad, as a recent mass spectrometry-based study also reported [21]. Although these substrates are modified from their native forms by truncation and by the addition of fluorophore and quencher moieties, our observations warrant further investigation to determine whether promiscuous cleavage of native substrates occurs under physiologically relevant conditions.
Figure 4. Cleavage of physiologically derived peptides.
Sequences are summarized in Table 2. Promiscuous cleavage was observed for all substrates except the bradykinin-derived substrate. Extent of cleavage is expressed as endpoint fluorescence fold change at 6 h. Dark bars denote cleavage by FAP, while light bars denote cleavage by POP. Error bars represent standard deviations. * p < 0.05 and ** p < 0.01 versus cleavage of the same substrate by the non-canonical enzyme.
Limitations
Several caveats should be noted when interpreting our data. First, the IQFP library we employed contains only three variable positions and is by design restricted to endopeptidase substrates. Also, the library lacks some residues that have been found in POP and FAP substrates, such as His and Trp, which interfere with fluorescence or may pose synthetic challenges in the IQFP library format. Finally, because library wells contain a mixture of IQFPs, an additional secondary deconvolution step is required to determine fine substrate specificity and to assess enzyme kinetics. However, these limitations are offset by the benefits of a concise yet unbiased library that can be screened in only 6 microplates. Another important consideration is common to all IQFP substrates – synthetic fluorophore-modified peptides may not recapitulate the folded structures of true endogenous substrates. Thus, mass spectrometry-based and computational studies [25] may be required to extend biochemical observations from synthetic substrates to physiologic ones. Nonetheless, libraries such as the one employed here can be used as a first step in the identification of selective probes and inhibitors to distinguish closely related enzymes.
Conclusion
In summary, we comparatively profiled the substrate specificities of POP and FAP and identified novel and selective substrates that will be useful as scaffolds for inhibitor design, probes for fundamental biochemical study, and triggers for controlled drug delivery.
Supplementary Material
Highlights.
We identified novel and selective substrates of prolyl oligopeptidase (POP) and fibroblast activation protein α (FAP), which may be used as scaffolds to design probes and inhibitors.
New insights into POP substrate specificity include a bias against charged residues at the P1′ and P2′ positions.
New FAP-specific substrates preferentially contain charged residues at the P1′ and P2′ positions and are entirely devoid of serine and threonine.
In synthetic internally quenched fluorogenic probes (IQFP) format, some physiologic substrates of FAP are promiscuously cleaved by POP.
Acknowledgments
This work was supported in part by grant R21AI08502 from the National Institute of Allergy and Infectious Diseases. SRI International’s Center for Advanced Drug Research was established with funding support from the Commonwealth of Virginia. The authors wish to acknowledge the encouragement and guidance of Dr. Walter Moos, Vice President of SRI Biosciences.
Abbreviations
- ACN
Acetonitrile
- APCE
Antiplasmin-cleaving enzyme
- DMSO
Dimethylsulfoxide
- DPA
Nb-(2,4-dinitrophenyl)-L-2,3-diaminopropionic acid
- DPPIV
Dipeptidyl peptidase IV
- FAP
Fibroblast activation protein
- IQFP
Internally quenched fluorogenic probes
- MALDI-TOF
Matrix assisted laser desorption/ionization – time of flight
- MCA
7-methoxycoumarin-4-acetic acid
- POP
Prolyl oligopeptidase
- ZPP
Z-prolyl prolinal
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at INSERTED BY EDITOR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drag M, Salvesen GS. Emerging principles in protease-based drug discovery. Nat Rev Drug Discov. 2010;9:690–701. doi: 10.1038/nrd3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Horsman JA, Mannisto PT, Venalainen JI. On the role of prolyl oligopeptidase in health and disease. Neuropeptides. 2007;41:1–24. doi: 10.1016/j.npep.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Watson DS, Jambunathan K, Askew DS, Kodukula K, Galande AK. Robust substrate profiling method reveals striking differences in specificities of serum and lung fluid proteases. Biotechniques. 2011;51:95–104. doi: 10.2144/000113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–9. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilk S, Orlowski M. Inhibition of rabbit brain prolyl endopeptidase by n-benzyloxycarbonyl-prolyl-prolinal, a transition state aldehyde inhibitor. J Neurochem. 1983;41:69–75. doi: 10.1111/j.1471-4159.1983.tb11815.x. [DOI] [PubMed] [Google Scholar]
- 6.Myohanen TT, Garcia-Horsman JA, Tenorio-Laranga J, Mannisto PT. Issues about the physiological functions of prolyl oligopeptidase based on its discordant spatial association with substrates and inconsistencies among mRNA, protein levels, and enzymatic activity. J Histochem Cytochem. 2009;57:831–48. doi: 10.1369/jhc.2009.953711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien P, O’Connor BF. Seprase: an overview of an important matrix serine protease. Biochim Biophys Acta. 2008;1784:1130–45. doi: 10.1016/j.bbapap.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Lee KN, Jackson KW, Christiansen VJ, Lee CS, Chun JG, McKee PA. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood. 2006;107:1397–404. doi: 10.1182/blood-2005-08-3452. [DOI] [PubMed] [Google Scholar]
- 9.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274:36505–12. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 10.Edosada CY, Quan C, Tran T, Pham V, Wiesmann C, Fairbrother W, Wolf BB. Peptide substrate profiling defines fibroblast activation protein as an endopeptidase of strict Gly(2)-Pro(1)-cleaving specificity. FEBS Lett. 2006;580:1581–6. doi: 10.1016/j.febslet.2006.01.087. [DOI] [PubMed] [Google Scholar]
- 11.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci U S A. 1990;87:7235–9. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf BB, Quan C, Tran T, Wiesmann C, Sutherlin D. On the edge of validation--cancer protease fibroblast activation protein. Mini Rev Med Chem. 2008;8:719–27. doi: 10.2174/138955708784567449. [DOI] [PubMed] [Google Scholar]
- 13.Collins PJ, McMahon G, O’Brien P, O’Connor B. Purification, identification and characterisation of seprase from bovine serum. Int J Biochem Cell Biol. 2004;36:2320–33. doi: 10.1016/j.biocel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Keane FM, Nadvi NA, Yao TW, Gorrell MD. Neuropeptide Y, B-type natriuretic peptide, substance P and peptide YY are novel substrates of fibroblast activation protein-alpha. FEBS J. 2011;278:1316–32. doi: 10.1111/j.1742-4658.2011.08051.x. [DOI] [PubMed] [Google Scholar]
- 15.Kraman M, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827–30. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DA, et al. A broad-spectrum fluorescence-based peptide library for the rapid identification of protease substrates. Proteomics. 2006;6:2112–20. doi: 10.1002/pmic.200500153. [DOI] [PubMed] [Google Scholar]
- 17.Noula C, Kokotos G, Barth T, Tzougraki C. New fluorogenic substrates for the study of secondary specificity of prolyl oligopeptidase. J Pept Res. 1997;49:46–51. doi: 10.1111/j.1399-3011.1997.tb01119.x. [DOI] [PubMed] [Google Scholar]
- 18.Gorrao SS, Hemerly JP, Lima AR, Melo RL, Szeltner Z, Polgar L, Juliano MA, Juliano L. Fluorescence resonance energy transfer (FRET) peptides and cycloretro-inverso peptides derived from bradykinin as substrates and inhibitors of prolyl oligopeptidase. Peptides. 2007;28:2146–54. doi: 10.1016/j.peptides.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Shan L, Marti T, Sollid LM, Gray GM, Khosla C. Comparative biochemical analysis of three bacterial prolyl endopeptidases: implications for coeliac sprue. Biochem J. 2004;383:311–8. doi: 10.1042/BJ20040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bordusa F, Jakubke HD. The specificity of prolyl endopeptidase from Flavobacterium meningoseptum: mapping the S’ subsites by positional scanning via acyl transfer. Bioorg Med Chem. 1998;6:1775–80. doi: 10.1016/s0968-0896(98)00145-x. [DOI] [PubMed] [Google Scholar]
- 21.Aggarwal S, Brennen WN, Kole TP, Schneider E, Topaloglu O, Yates M, Cotter RJ, Denmeade SR. Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry (Mosc) 2008;47:1076–86. doi: 10.1021/bi701921b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KN, Jackson KW, Terzyan S, Christiansen VJ, McKee PA. Using substrate specificity of antiplasmin-cleaving enzyme for fibroblast activation protein inhibitor design. Biochemistry (Mosc) 2009;48:5149–58. doi: 10.1021/bi900257m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11:257–66. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez A, Tarrago T, Giralt E. Low molecular weight inhibitors of Prolyl Oligopeptidase: a review of compounds patented from 2003 to 2010. Expert Opin Ther Pat. 2011;21:1023–44. doi: 10.1517/13543776.2011.577416. [DOI] [PubMed] [Google Scholar]
- 25.Boyd SE, Garcia de la Banda M, Pike RN, Whisstock JC, Rudy GB. PoPS: A computational toll for modeling and prediciting protease specificity. J Bioinform comput Biol. 2005;3:551–585. doi: 10.1142/s021972000500117x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.