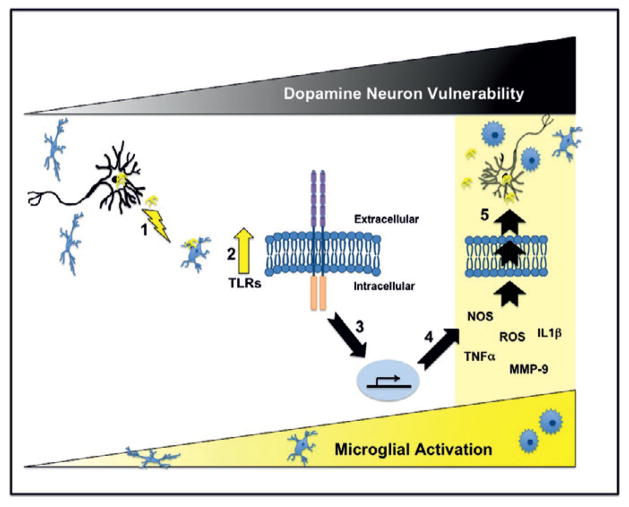
Fig. 2.
α-Synuclein incites microglial activation via toll-like receptor activation. This schematic diagram depicts our current hypothesis that α-synuclein released from neurons and/or presynaptic terminals (e.g., activity dependent release or as a result of ongoing neurodegeneration) activates microglia [1]. We have previously demonstrated that α-synuclein-mediated activation of microglia results in increased expression of TLRs (2; lightning bolt; Table 1 and ref. [12]). Importantly TLRs can mediate downstream pathways that result in the translocation of NFκB to the nucleus, which in turn causes increased expression of proinflammatory molecules (3 & 4; Table 1) that are detrimental to dopamine neurons (5; black triangle “Dopamine Neuron Vulnerability”). Microglial activation is often accompanied by a change in morphology from highly branched cells (ramified) to rounded cells with little branching (amboid) as indicated in the lower yellow triangle. Our data indicates that 24-hours post-exposure α-synuclein-directed microglial activation results in increased expression of TNF-α, NOS, IL-1β, MMP-9, ROS and morphological changes consistent with enhanced classical inflammation (Table 1 and Figure 1). If this proinflammatory milieu continues progressive death of dopamine neurons releasing α-synuclein would ensue followed by continued activation of microglia. However, since TLRs can also promote cell growth and survival it will be important in future studies to establish the specific TLRs that are altered by cognate α-synuclein conformers and subsequently to determine the exact downstream TLR pathways activated (e.g., MyD88 and/or PI3K) so that targeted therapies can be developed.