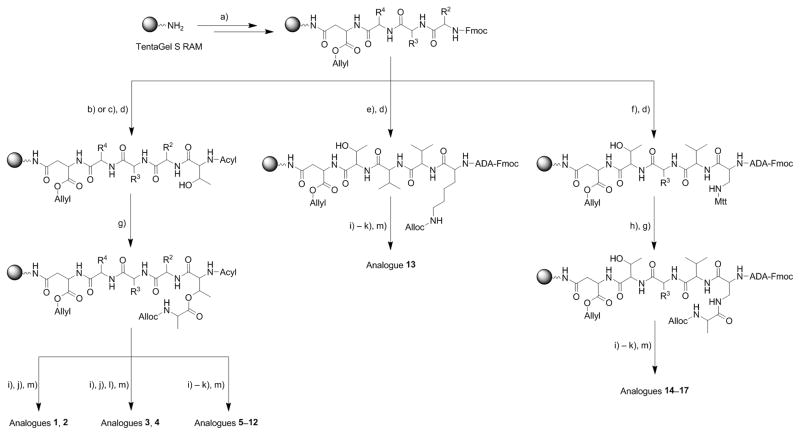
Scheme 1.
Synthesis of fusaricidin A/LI-F04a analogs 1–17. Reagents and conditions: a) Fmoc-D-Asp-OAllyl, and Fmoc-AA-OH, standard Fmoc-SPPS deprotection and coupling protocols; b) Ac-Thr-OH, standard Fmoc-SPPS deprotection and coupling protocols; c) Fmoc-Thr-OH, standard Fmoc-SPPS deprotection and coupling protocols; d) Fmoc-ADA-OH, standard Fmoc-SPPS deprotection and coupling protocols; e) Fmoc-Lys-OH, standard Fmoc-SPPS deprotection and coupling protocols; f) Fmoc-Dap(Mtt)-OH, standard Fmoc-SPPS deprotection and coupling protocols; g) Alloc-D-Ala-OH or Alloc-Gly-OH (4 eq.), DIC (4 eq.), DMAP (0.2 eq.), CH2Cl2, r.t., 18 h; h) 1% TFA/CH2Cl2 (v/v), r.t. 30 min.; i) Pd(Ph3P)4 (0.1 eq.), HN(CH3)2·BH3 (4 eq.), CH2Cl2, r.t. 2×10 min.; j) PyBOP/HOBt/DIEA (2/2/6 eq.), DMF, r.t. 18 h; k) 20% piperidine/DMF (v/v), r.t., 25 min.), N,N-bis(tert-butoxycarbonyl)thiourea (3 eq.), 2-chloro-1-methylpyridinium iodide (3 eq.), TEA (4 eq), DMF, r.t. 18 h; l) 20% piperidine/DMF (v/v), r.t., 25 min.); m) TFA/TIA/H2O=95:2.5:2.5 (v/v/v), r.t. 3 h.