Abstract
Eph receptor tyrosine kinases and their Ephrin ligands represent an important signaling system with widespread roles in cell physiology and disease. Receptors and ligands in this family are anchored to the cell surface; thus Eph/Ephrin interactions mainly occur at sites of cell-to-cell contact. EphB4 and EphrinB2 are the Eph/Ephrin molecules that play essential roles in vascular development and postnatal angiogenesis. Analysis of expression patterns and function has linked EphB4/EphrinB2 to endothelial cell growth, survival, migration, assembly, and angiogenesis. Signaling from these molecules is complex, with the potential for being bidirectional, emanating both from the Eph receptors (forward signaling) and from the Ephrin ligands (reverse signaling). In this review, we describe recent advances on the roles of EphB/EphrinB protein family in endothelial cell function and outline potential approaches to inhibit pathological angiogenesis based on this understanding.
I. INTRODUCTION
The Eph receptor family, which includes 14 members, constitutes the largest family of tyrosine kinase receptors in mammals. Eph receptors and their Ephrin (Eph receptor interacting) ligands form a system of cell communication with widespread roles in physiology and disease. In mammals, there are nine EphA (EphA1–8, and EphA10) receptors, which promiscuously bind five glycosylphosphatidylinositol (GPI)-linked EphrinA ligands and five EphB (EphB1–4 and EphB6) receptors, which promiscuously bind three transmembrane EphrinB ligands (Chrencik et al., 2006; Egea and Klein, 2007; Kullander and Klein, 2002; Pasquale, 2010). There are some exceptions: EphA4 can bind both A-type and most B-type Ephrins, EphB2 can bind EphrinA5, and EphB4 essentially binds only EphrinB2 (Table I). Typically, Eph receptor–Ephrin ligand interactions occur at the cell’s surface at sites of cell-to-cell contact and result in bidirectional signaling from the Eph receptor (forward signaling) and from the Ephrin ligand (reverse signaling) (Chrencik et al., 2006; Egea and Klein, 2007; Pasquale, 2010).
Table I.
Listing of EphA, EphrinA, EphB, and EphrinB Proteins
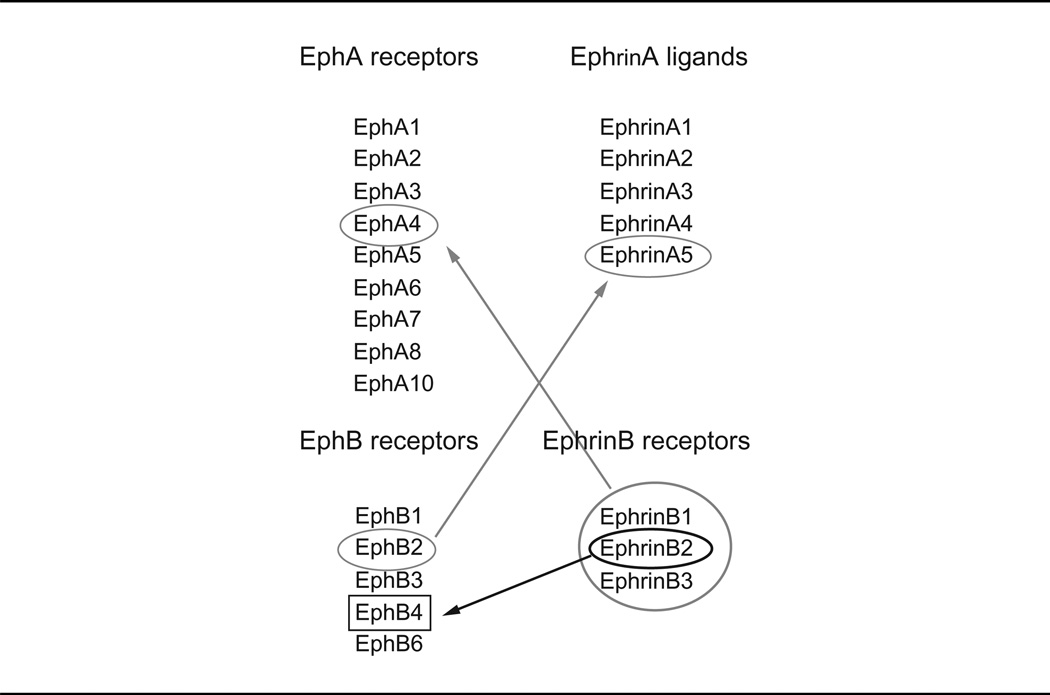 |
Although EphrinA ligands generally bind to EphA receptors, there are exceptions noted by the red arrows. Although EphrinB ligands generally bind all EphB receptors, EphrinB2 essentially binds only to EphB4.
EphrinB2 and its receptor EphB4 play critical roles in the development of the vascular system and contribute to the function of the adult vasculature (Pitulescu and Adams, 2010). Knockout mice lacking EphrinB2 or EphB4 expression and mice with deletion of EphrinB2 targeted to the endothelial cells display a severely compromised vascular system and die at midgestation (Adams et al., 1999; Gerety and Anderson, 2002; Gerety et al., 1999). The targeted deletion ofEphrinB2 inmural cells (pericytes and smoothmuscle cells) leads to diffuse tissue edema, hemorrhaging, and perinatal death of the mice (Foo et al., 2006). EphrinB is phosphorylated in angiogenic blood vessels, and inhibition of phosphorylation-dependent or PDZ-dependent signaling down-stream of EphrinB ligands prevents endothelial cell sprouting and the proper assembly of endothelial cells with other endothelial cells and with pericytes (Salvucci et al., 2006, 2009; Sawamiphak et al., 2010b;Wang et al., 2010b).
In this review, we will present current information on structural features, signaling mechanisms, and expression and function of the EphB/EphrinB proteins in the context of blood vessel physiology and pathology.
II. STRUCTURAL FEATURES AND INTERNALIZATION
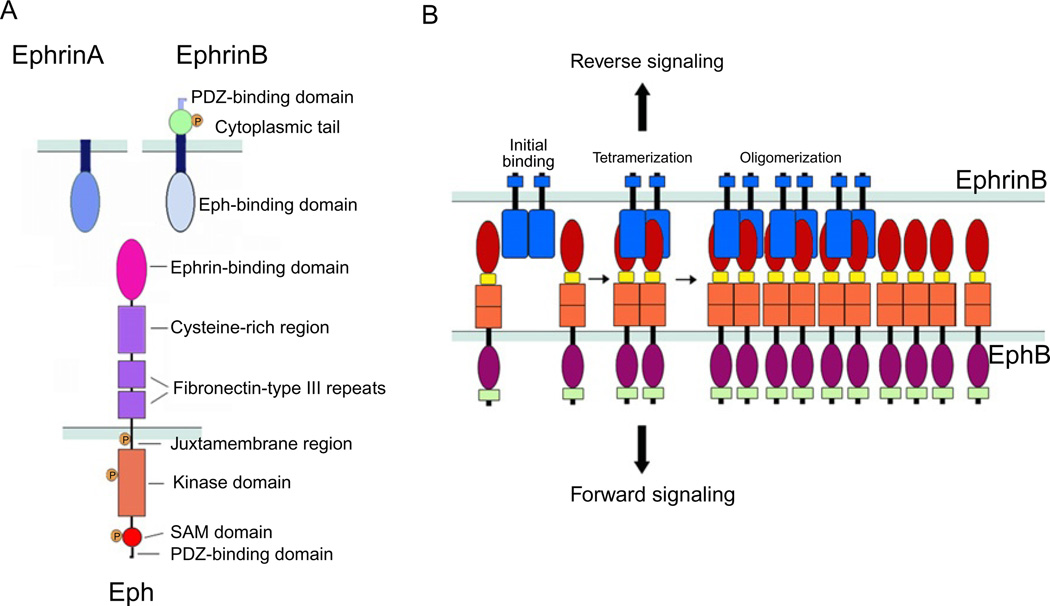
The extracellular portion of Eph receptors (both A and B families) includes a globular, ligand-binding domain; a cysteine-rich EGF-like region; and two fibronectin-type III repeats (Himanen and Nikolov, 2003; Himanen et al., 2001; Toth et al., 2001). The intracytoplasmic portion consists of a short membrane-proximal region, a tyrosine kinase domain, a sterile alpha motif (SAM) protein–protein interaction domain, and a PDZ-binding C-terminal motif (Fig. 1A; Chrencik et al., 2006). The B Ephrins display an extracellular Eph receptor-binding domain, a single-pass transmembrane region, a short intracellular domain with several sites for tyrosine and serine phosphorylation, and a C-terminal PDZ-binding motif (Fig. 1A; Chrencik et al., 2006).
Fig. 1.
(A) Schematic representation of the domain structure and binding interfaces of Ephrins and Eph receptors. EphrinA ligands are attached to the cell surface through a glycosylphosphatidylinositol (GPI)-anchor; the extracellular domain contains an Eph receptor-binding domain that is connected to the transmembrane segment. EphrinB ligands are transmembrane proteins with an extracellular Eph receptor-binding domain connected to a transmembrane segment, which is followed by a short intracellular domain. The Eph receptors include an extracellular domain composed of an Ephrin-binding domain, a cysteine-rich segment that contains an epidermal growth factor (EGF)-like motif, and two fibronectin-type III domains; and a cytoplasmic region that contains a juxtamembrane region, the kinase domain, a sterile a-motif (SAM), and a binding site for PDZ-containing proteins. (B) Representation of initial binding of cell surface Eph and Ephrin molecules to form heterotetramers, which initiate signaling, and subsequent oligomerization to form large receptor/ligand clusters that expand laterally through hemophilic interactions between Eph receptors.
Initial high-affinity interaction between the Eph receptor globular domain and a protruding loop of EphrinB leads to conformational changes of receptor and ligand, and the formation of heterodimers of an Eph receptor with an Ephrin molecule (Himanen et al., 2001; Toth et al., 2001). Other residues in the Eph receptor contribute to a lower-affinity binding interface for much of the extracellular region of EphrinB2, leading to the association of two homodimers to form a tetrameric complex comprising two Eph receptors and two Ephrin molecules (Chrencik et al., 2006; Himanen and Nikolov, 2003). Eph/Ephrin tetramers can aggregate into larger clusters through several low-affinity Eph–Eph and Ephrin–Ephrin interactions identified in the extracellular domains (Fig. 1B; Smith et al., 2004).
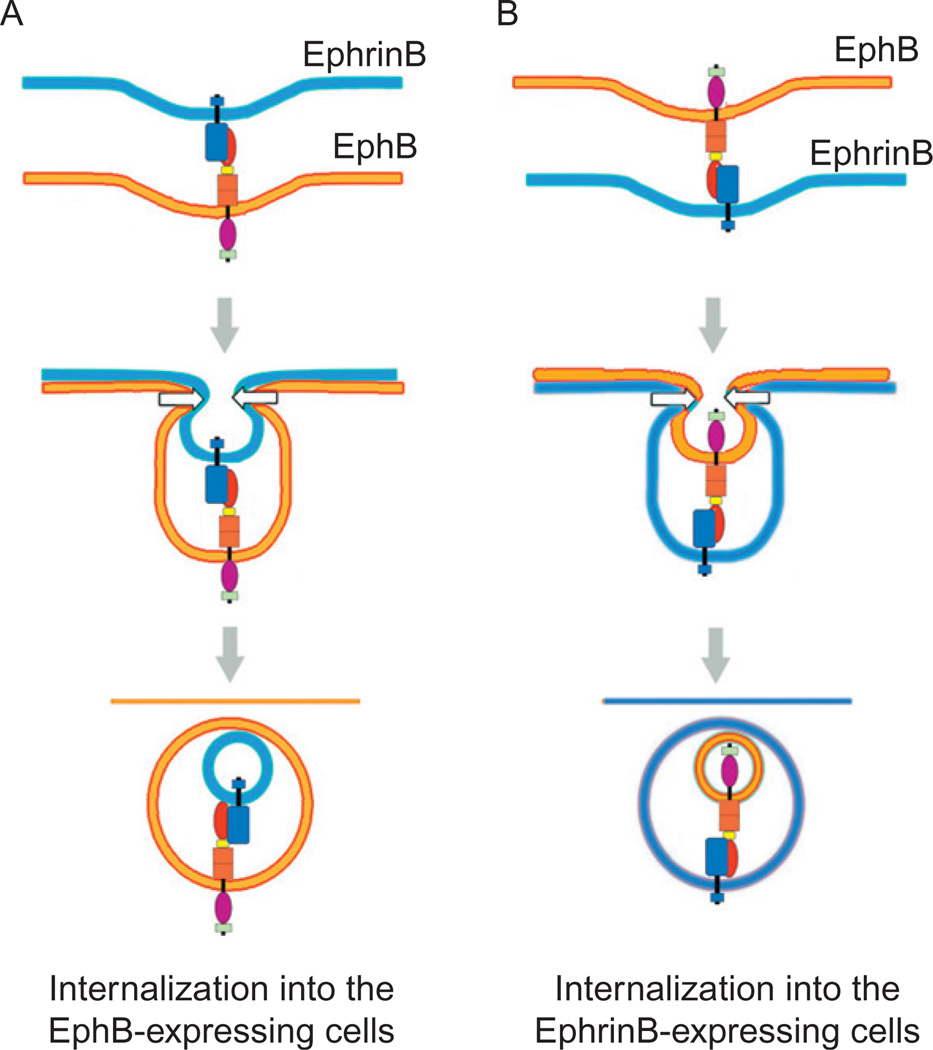
One of the reported consequences of high-affinity interactions involving EphB and EphrinB expressed on adjacent cells is internalization of both molecules into Eph or Ephrin-expressing cells (Lauterbach and Klein, 2006; Mann et al., 2003; Marston et al., 2003; Zimmer et al., 2003). This process provides a mechanism for removal of Eph receptors and ligands from the cell surface and termination of receptor/ligand adhesive interactions, which may explain the occurrence of cell repulsion (Egea and Klein, 2007). EphB–EphrinB complexes, detected in intracellular vesicles as full-length proteins, are believed to be the result of trans-endocytosis from one cell to the adjacent cell (Fig. 2). The direction of endocytosis seems to be dependent on the direction of signaling. For example, if a cell expressing EphB2 contacts a cell expressing EphrinB1 in which the C-terminal domain is truncated, endocytosis of the complex occurs preferentially into the EphB2-expressing cell (Zimmer et al., 2003). By contrast, if EphB2 is signaling-deficient, the internalization occurs in the EphrinB1-expressing cell. If, however, both EphB and EphrinB are signaling-impaired, internalization of the Eph/Ephrin complex is not observed (Zimmer et al., 2003). Although the biochemical basis for internalization of Eph/Ephrin is currently poorly defined, EphB1 was found to be associated with caveolin-1, suggesting the potential involvement of caveolae (Vihanto et al., 2006), and EphrinB1 was found to be associated with clathrin-coated vesicles, suggesting a clathrin-dependent mechanism (Vihanto et al., 2006). Once internalized, signaling (reverse or forward) has been shown to persist within the recipient cell (Marston et al., 2003).
Fig. 2.
Interaction of EphrinB with EphB can lead to (A) trans-endocytosis in the direction of the receptor (forward endocytosis) or (B) trans-endocytosis in the direction of the ligand (reverse endocytosis). Both processes lead to the internalization of full-length ligand and receptor.
In addition to undergoing internalization of themselves, EphB and EphrinB can promote the internalization of the surrounding membrane and other proteins. For example, EphB signaling has been shown to modulate the clathrin-mediated endocytosis of amino-3-hydroxy-5-methyl-4-isoxazolpropionic acid-type glutamate receptors in neurons (Irie et al., 2005), and EphrinB signaling has been reported to promote the internalization of VEGFR2 and VEGFR3 in endothelial cells (Sawamiphak et al., 2010b; Wang et al., 2010b).
III. BIDIRECTIONAL SIGNALING
Eph receptor signaling induced by Ephrin binding (forward signaling) is initiated by autophosphorylation and Src family kinases-mediated phosphorylation of the intracellular tyrosine residues, resulting in the activation of the tyrosine kinase catalytic domain (Kalo and Pasquale, 1999; Knoll and Drescher, 2004; Kullander and Klein, 2002; Murai and Pasquale, 2003). Once the Eph receptors are phosphorylated, adaptor proteins containing Src-homology 2 (SH2) domains can bind and initiate phosphorylation of downstream substrates (Brantley-Sieders et al., 2004; Kalo and Pasquale, 1999; Murai and Pasquale, 2003; Wybenga-Groot et al., 2001; Zisch et al., 2000). Activated Eph receptors can also mediate other protein–protein interactions via the SAM and PDZ-binding motifs, which contribute to signaling (Chrencik et al., 2006; Kullander and Klein, 2002; Noren and Pasquale, 2004). Key components of Eph signaling are the Rho family of GTPases, including RhoA, Cdc42, and Rac, which are involved in the regulation of the actin cytoskeleton and cell shape, movement, and adhesion (Klein, 2009; Ogita et al., 2003; Shamah et al., 2001). Rho GTPases shuttle between an inactive (GDP-bound) and a signaling-active (GTP-bound) state, and this transition is regulated by guanidine nucleotide exchange factors (GEFs), which activate the Rho GTPases (Holland et al., 1997). Phosphorylated Eph receptors have been shown to associate and activate a number of Rho GEFs, including Vav2, Tiam, Kalirin, and Intersectin (Cowan et al., 2005; Klein, 2009; Murai and Pasquale, 2005; Sahin et al., 2005). In addition, phosphorylated EphB has been shown to induce the ubiquitination and degradation of the GEF Ephexin 5, which binds to EphB and inhibits RhoA activity (Margolis et al., 2010).
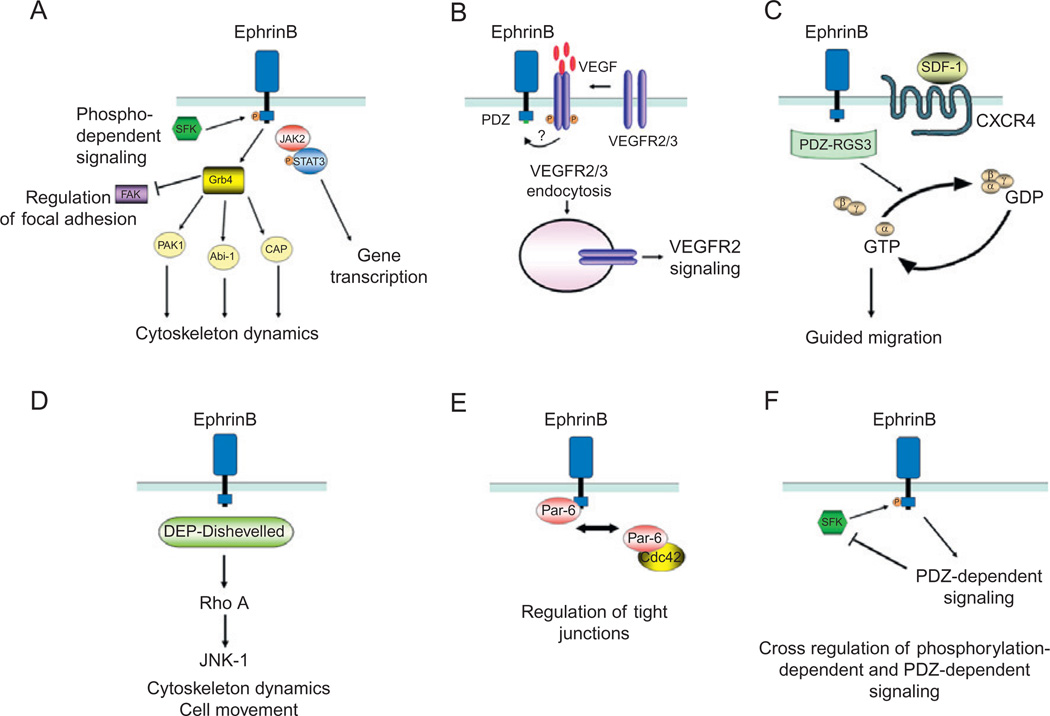
Unlike Eph receptors, B Ephrins do not possess intrinsic catalytic activity, and thus rely on the recruitment of signaling molecules to signal. EphrinB signaling upon receptor engagement and clustering (reverse signaling) is initiated by the recruitment and activation of Src family kinases, which phosphorylate specific tyrosine residues in the intracytoplasmic domain of B Ephrins (Palmer et al., 2002; Fig. 3A). The activation of Src kinases is dependent upon metalloproteinase and presenilin-1-γ-secretase-mediated enzymatic cleavage of an intracellular peptide of EphrinB that binds to Src inhibiting its association with the inhibitory protein Csk (Georgakopoulos et al., 2006). Phosphorylated EphrinB provides a docking site for the SH2 domain-containing adaptor protein Grb4 and the signal transducer and activator of transcription STAT3 (Bong et al., 2007; Cowan and Henkemeyer, 2001; Segura et al., 2007; Tanaka et al., 2003; Xu and Henkemeyer, 2009). The EphrinB–Grb4 complex results in the activation of focal adhesion kinase (FAK) catalytic activity and recruitment of the G-protein-coupled receptor kinase interacting protein (GIT) 1 (Cowan and Henkemeyer, 2001; Segura et al., 2007). Grab4 can also associate with other proteins involved in cytoskeleton regulation, including dynamin, Cbl-associated protein (CAP/ponsin), the Abl-interacting protein (Abi-1), and p21-activated kinase (PAK1; Cowan and Henkemeyer, 2001). Upon binding to EphrinB, STAT3 undergoes Jak-2-dependent phosphorylation and migrates to the nucleus, where it regulates a variety of target genes, thus linking membrane EphrinB to the nucleus (Bong et al., 2007).
Fig. 3.
Mechanisms of EphrinB reverse signaling. (A) EphrinB activation by EphB receptor leads to the recruitment of Src family kinases (SFKs) that phosphorylate EphrinB intracellular domain at tyrosine residues. The adaptor molecule Grb4, which contains a Src-homology-2 (SH2) domain, is recruited to the phosphorylated EphrinB and initiates a number of signaling events that regulate cytoskeleton dynamics and focal adhesions. The phosphorylated EphrinB can also recruit the Jak2/STAT3 complex; the phosphorylated STAT3 translocates to the nucleus regulating expression of target genes. (B) Activated EphrinB regulates the internalization of VEGFR2 and VEGFR3 through PDZ-mediated binding of yet unknown protein(s). After VEGF binding, VEGFR is phosphorylated; signaling from activated VEGFR requires internalization, which is positively regulated by EphrinB signaling. (C) RGS3 is a PDZ-containing protein that constitutively binds to EphrinB and links signaling from the G-protein-coupled receptor CXCR4 to EphrinB. CXCR4 signaling in response to the ligand SDF1 is induced by the dissociation of Gβγ and GTP-Gα subunits. PDZ-RGS3 can inhibit CXCR4 signaling by enhancing the GTPase activity of the Gα subunit, resulting in the reformation of the inactive heterodimeric CXCR4 receptor. (D) The scaffold protein Dishevelled binds EphrinB through its DEP domain and mediates signaling via the Rho small GTPase pathway. (E) The scaffold protein Par6 associates with EphrinB resulting in the loss of tight junctions. PAR6 forms a complex with activated apical protein kinase C (aPKC) and Cdc42-GTP; the complex localizes to the apical cell junctions where it regulates tight junctions. EphrinB1 can compete with Cdc42 for binding to Par6 and thus reduce tight junctions. (F) Cross-regulation between EphrinB phosphorylation- and PDZ-dependent signaling pathways. The phosphatase PTB-BL, recruited through its PDZ domain to EphrinB cytoplasmic domain, inactivates Src and dephosphorylates EphrinB.
EphrinB can also become phosphorylated in the absence of receptor engagement. In the chicken retina, FGF can induce tyrosine phosphorylation of endogenous EphrinB, presumably through the coexpressed FGF receptor (Chong et al., 2000; Lee et al., 2009). In fibroblasts, activation of the PDGF receptor induces EphrinB1 phosphorylation (Bruckner et al., 1997), as does activation of Tie2 by angiopoietin-1 in endothelial cells (Adams et al., 1999).
A number of studies have highlighted the importance of tyrosine phosphorylation-independent signaling by EphrinB originating from protein–protein interactions involving the C-terminal PDZ-binding motif (Egea and Klein, 2007; Lin et al., 1999; Noren and Pasquale, 2004). Genetic studies have shown that knock-in mice expressing a mutant EphrinB2, in which the five tyrosines have been substituted to prevent phosphorylation, are only modestly affected (Makinen et al., 2005). In contrast, knock-in mice expressing a mutant EphrinB2, in which the PDZ-interacting domain was disrupted, present major defects in lymphatic vascular (Makinen et al., 2005) and retinal vessel development (Sawamiphak et al., 2010b).
Biochemical studies have provided examples of phosphorylation-independent EphrinB signaling. In one such example, both the WTand phosphorylation-deficient EphrinB2 proteins were reported to similarly regulate the internalization of VEGFR2, whereas the PDZ-mutant EphrinB2 did not. This observation suggested that the binding of activated EphrinB2 to a PDZ-containing protein (yet to be identified) mediates the internalization of VEGFR2 or VEGFR3 (Sawamiphak et al., 2010b; Fig. 3B). In another example, the EphrinB cytoplasmic domain was reported to bind to the PDZ-containing RGS3 (regulator of G-protein signaling) protein, which can regulate G-protein-coupled receptor signaling (Lu et al., 2001; Fig. 3C). Another PDZ-dependent signaling pathway involves the interaction between EphrinB and PAR-3, a scaffold protein member of the Par (partitioning defective) complex (Lin et al., 1999), which has been shown to stimulate Cdc42-induced Rac activation (Lin et al., 1999; Nishimura et al., 2005). Dishevelled, a scaffold protein that plays important roles in the Wnt signaling pathway, has been shown to bind to unphosphorylated EphrinB as well as to tyrosine-phosphorylated EphrinB, a binding which is mediated by Grb4 (Boutros and Mlodzik, 1999; Tanaka et al., 2003). The phosphorylation-independent EphrinB1 binding of Dishevelled was found to require the presence of the DEP (Dishevelled, Egl–10, Pleckstrin), but not the PDZ domain, of Dishevelled (Lee et al., 2006). Functionally, the binding of Dishevelled to EphrinB1 has been shown to mediate signals through the Rho small GTPase pathway (Lee et al., 2006; Tanaka et al., 2003; Fig. 3D). More recently, Par-6, another member of the Par protein family, was found to bind to EphrinB1 at the C-terminus through a non-PDZ-binding motif (Lee et al., 2009). This Par-6/EphrinB1 complex was found to compete with the small GTPase Cdc42 for binding to Par-6, providing evidence that Par-6 is a mediator of EphrinB1 signaling (Lee et al., 2009; Fig. 3E). Recent genetic experiments have further suggested the existence of phosphorylation and PDZ-independent EphrinB signaling, potentially mediated by Grb4 docking via the EphrinB SH3 domain or the association with Dishevelled (Dravis and Henkemeyer, 2011).
There is evidence of cross-regulation between phosphorylation and PDZ-dependent EphrinB signaling. For example, the tyrosine phosphatase PTB-BL, which contains a PDZ motif, is recruited to the active EphrinB and can negatively regulate EphrinB phosphorylation and Src activity (Palmer et al., 2002; Fig. 3F).
Many of the signaling pathways initiated by EphB/EphrinB signaling described above have been identified in endothelial cells and have been linked to various endothelial cell functions (Chrencik et al., 2006). EphB4 signaling has been shown to activate the Akt, PI3K (phosphatidylinositol 3-kinase), and the MAPK (mitogen activated protein kinases) pathways promoting endothelial cell proliferation and migration (Steinle et al., 2002). EphB2 and EphB4 signaling was found to enhance SDF1/CXCL12-induced Akt phosphorylation (Salvucci et al., 2006). EphrinB phosphorylation was reported to transiently activate Src family kinases, which are positive regulators of EphrinB phosphorylation, inducing endothelial cell sprouting (Palmer et al., 2002; Salvucci et al., 2009). Also, EphrinB phosphorylation was reported to induce Jak2-dependent STAT3 phosphorylation, contributing to endothelial cell assembly onto extracellular matrix (Salvucci et al., 2009). EphrinB2 stimulation resulted in the activation of the PI3K and MAPK pathways in vascular endothelial cells promoting their proliferation (Steinle et al., 2003) and migration (Maekawa et al., 2003). EphrinB1 activation promoted JNK phosphorylation through interaction of the C-terminal domain with PDZ-containing proteins enhancing endothelial cell attachment and migration (Huynh-Do et al., 2002).
Studies in vivo have revealed the importance of EphrinB2–PDZ interaction for reverse signaling underlying the development of lymphatic vessels (Makinen et al., 2005), and the regulation of VEGFR2 and VEGFR3 internalization and signaling in blood vessels thereby modulating endothelial cell growth, survival, and migration (Sawamiphak et al., 2010b). Although the biochemical basis for signaling from EphrinB/PDZ interactions has not been clarified in endothelial cells, the contribution of Rho family GTPases might be important in this context, analogous to the situation in neuronal cells and other cells (Egea and Klein, 2007; Lee et al., 2006; Noren and Pasquale, 2004; Tanaka et al., 2003, 2004).
IV. ROLE OF B-TYPE EPHS AND EPHRINS IN VASCULAR DEVELOPMENT
The formation of blood vessels starts early during development of the mouse embryo, and the presence of functional vessels becomes necessary around midgestation. Two processes contribute to the development of vascular structures: vasculogenesis, the process by which endothelial cell precursors (angioblasts) form endothelial tubules, which contributes to the formation of the primary vascular plexus in the yolk sac, and angiogenesis, the process by which an existing vasculature forms sprouts and generates new vessels, thus expanding and remodeling an existing vascular bed, which contributes to the formation of most vessels during development and after birth.
Genetic experiments in mice have demonstrated that the global deletion of EphB4 or EphrinB2 leads to embryonic death with marked defects in the “remodeling” of the primary vascular plexus, suggesting that these molecules are critical to early vascular development (Adams et al., 1999; Gerety et al., 1999;Wang et al., 1998). Consistent with this interpretation, deletion of EphrinB2 targeted to the developing vasculature produced a similar phenotype (Gerety and Anderson, 2002). The phenotype of EphB4-null or EphrinB2-null mice was indistinguishable, suggesting that forward signaling, reverse signaling, or a combination of forward and reverse signaling is necessary for normal vascular development. Mutant mice, in which the carboxy-terminal cytoplasmic tail of EphrinB2 was truncated (residues 264–336), displayed a very similar vascular phenotype to that of EphrinB2-null mice (Adams et al., 2001), suggesting that EphrinB2 reverse signaling is critical to early vascular development. A subsequent study using the same EphrinB2 C-terminal mutation in a different construct concluded that the cytoplasmic domain of EphrinB2 is not essential for vascular development as the mice were born live (Cowan et al., 2004). This study suggested that the discordant results were attributable to differences in trafficking of the mutant EphrinB2 to the cell surface and that the defective vascular development was attributable to defective stimulation of Eph signaling (Cowan et al., 2004). Nonetheless, cardiovascular development was compromised also in these mice, suggesting that EphrinB2 reverse signaling is critical at later stages of cardiovascular development (Cowan et al., 2004). Other genetic experiments examined the relative signaling contribution of phosphorylation and the PDZ-binding domain of EphrinB2 to vascular development (Makinen et al., 2005). Knock-in mice expressing a mutant EphrinB2, in which the conserved tyrosine residues were mutated to prevent phosphorylation, had no appreciable blood vascular defects (Makinen et al., 2005). Similarly, knock-in mice expressing a PDZ-mutant EphrinB2 were born normally without apparent blood vascular defects (Makinen et al., 2005). However, these EphrinB2 PDZ-mutant knock-in mice exhibited marked defects in lymphatic vessel development, whereas the phosphorylation-deficient knock-in mice had only minor defects in the lymphatic vessels (Makinen et al., 2005). Additional experiments using EphrinB2 PDZ-mutant mice concluded that EphrinB2 signaling is required for the normal development of retinal vessels (Sawamiphak et al., 2010b). Together, these results suggested that EphrinB2 reverse signaling mediated by the PDZ-binding domain is a critical contributor to lymphatic and retinal blood vessel development (Makinen et al., 2005; Sawamiphak et al., 2010b). EphrinB2-targeted deletion in pericytes and smooth muscle cells caused perinatal lethality associated with developmental defects in small-diameter blood vessels, which were not properly covered with smooth muscle cells/pericytes (Foo et al., 2006). Similar to the observations in mutant mice with a targeted deletion of EphrinB2 in the endothelium, overexpression of EphrinB2 in the endothelium during early development caused marked vascular defects and premature death at midgestation, providing evidence for a dosage-dependent function of EphrinB2 during vascular development (Wang et al., 2010b).
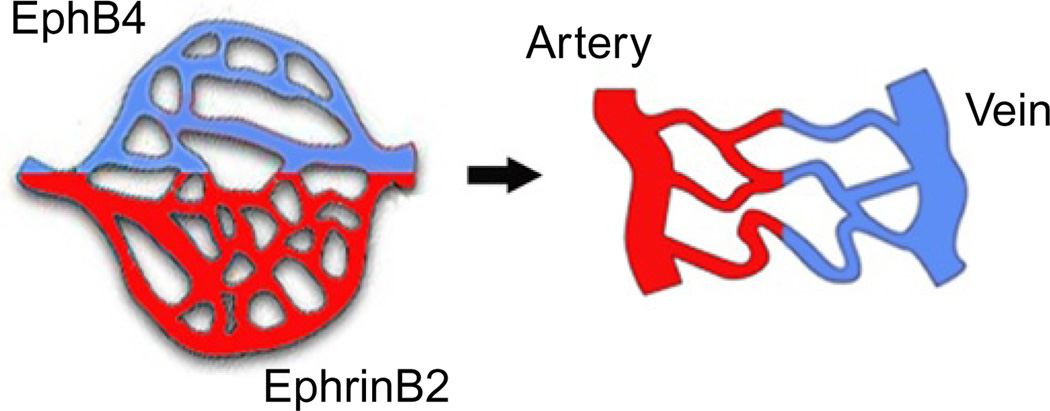
Several studies have revealed the importance of EphB4 and EphrinB2 in the determination of arterial–venous fate of endothelial cells (Adams et al., 1999; Gerety et al., 1999; Wang et al., 1998; Zhong et al., 2001; Fig. 4). Studies in zebrafish demonstrated that angioblasts migrating to the trunk (where they form the dorsal aorta and the cardinal vein) are predetermined to either an arterial or a venous fate based on their selective expression of the arterial marker EphrinB2 or venous marker EphB4. Initially, angioblasts assemble into a single precursor vessel from which the venous-fated, EphB4-expressing endothelial cells migrate and segregate to form the cardinal vein, whereas the EphrinB2-expressing cells do not move ventrally (Herbert et al., 2009). Various approaches to limit EphrinB2 or EphB4 function have established that this process of cell segregation is regulated by Eph/Ephrin interactions (Herbert et al., 2009). For example, if EphrinB2 expression is silenced by morpholinos or if EphrinB2 signaling is prevented by a C-terminal EphrinB2 deletion, the dorsal aorta contains very few cells. Conversely, if EphB4 is targeted with morpholinos, the cardinal vein is depleted of cells.
Fig. 4.
A role for EphB4 and EphrinB2 in arterial and venous fate determination. In the primitive vascular plexus, presumptive arterial and venous territories are marked by the distinctive expression of EphrinB2 or EphB4, prior to the formation of EphrinB2+ arteries and EphB4+ veins.
V. ANGIOGENESIS AND SPROUTING ANGIOGENESIS
Several mechanisms have been proposed to underlie the process of angiogenesis, that is, the formation of new vessels from existing vessels, including sprouting, elongation/widening, incorporation of circulating endothelial precursors, and formation of a lumen. There is evidence for a critical contribution of EphB4 and EphrinB2 function in several steps in angiogenesis.
EphrinB2 is expressed in the endothelium and mural cells of adult arteries, arterioles, and capillaries in many tissues, and levels of EphrinB2 expression increase in angiogenic vessels under physiological and pathological conditions (Gale et al., 2001; Korff et al., 2008; Shin et al., 2001). EphrinB is broadly phosphorylated in angiogenic vessels of the retina, skin wounds, and tumor vessels, but not in the resting endothelium (Salvucci et al., 2009). EphB4 is expressed most prominently in endothelial cells of venous derivation (Wang et al., 1998). This pattern of segregated expression of EphrinB2 and EphB4 has raised questions about the sites and extent of receptor/ligand interactions, since they would be limited to arterial/vein boundaries. However, expression of EphrinB2 and EphB4 partially overlaps in retinal vessels (Wang et al., 2010b), and endothelial cells derived from many sources, including the umbilical vein, human aorta, and dermal microvasculature, were found to express EphrinB2 and EphB4. This suggested that EphrinB2 and EphB4 have much broader avenues for interaction than previously appreciated (Gale et al., 2001; Salvucci et al., 2006; Shin et al., 2001). In addition, certain tumor cells express EphB4 and other members of the B- and A-type receptors, providing an opportunity for functional interactions with angiogenic tumor vessels that express EphrinB2 (Fukai et al., 2008; Noren et al., 2006; Zhuang et al., 2010).
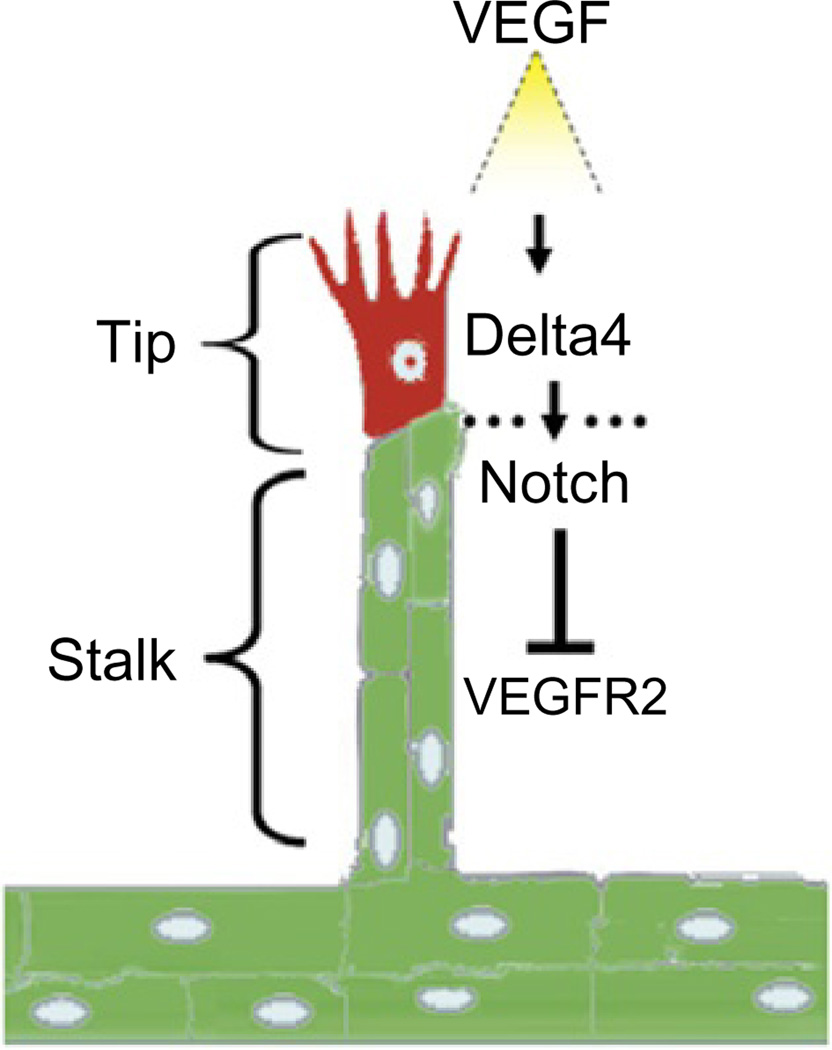
Angiogenic sprouting is characterized by the appearance of a pioneering endothelial cell, identified as a “tip” cell, which forms filopodial extensions and is locally invasive and motile in response to surrounding VEGF-A gradients (Gerhardt et al., 2003; Ferrara et al., 2003; Ruhrberg et al., 2002; Fig. 5). Other endothelial cells, named “stalk” cells, follow the tip cell and form the extending sprout at the base of the stalk (Gerhardt et al., 2003). The Notch and VEGF signaling pathways play critical roles in determining the distinctive roles of tip and stalk cells. Tip and stalk cells have both been shown to be responsive to local VEGF-A gradients (Ferrara et al., 2003), but this responsiveness appears to be modulated by opposing functions of the Notch ligands Dll4 and Jag1 (Benedito et al., 2009). VEGF-A induces the expression of the Notch ligand Dll4 in tip cells, which interacts with Notch1/4 expressed on the adjacent stalk cells reducing their expression of VEGFR2 and thus limiting stalk cell responsiveness to VEGF-A (Lobov et al., 2007; Suchting et al., 2007). A second Notch ligand, Jag1, is expressed by stalk cells and serves to stimulate Notch1/4 on tip cells contributing to their responsiveness to VEGF-A (Benedito et al., 2009). Another potential regulator of tip and stalk cell function is soluble VEGFR1. Stalk cells produce soluble VEGFR1, which neutralizes VEGF-A by competing with receptor binding, and may thus further limit or modulate responses to VEGF-A in the growing sprout (Chappell et al., 2009).
Fig. 5.
Angiogenesis is often initiated by the sprouting of endothelial cells from existing vessels. The leading cell in the sprout, the so-called tip cell, is characterized by filopodia extensions; the cells at the base of the sprout, the so-called stalk cells, are proliferating. VEGF/VEGFR2, the Notch ligands Delta4 and Notch signaling participate in orchestrating sprouting angiogenesis.
Recent studies have outlined an important role of EphrinB2 signaling in sprouting angiogenesis, particularly in the regulation of tip cell function. Developing retinal vessels grow by sprouting (Gariano and Gardner, 2005) and widely express EphrinB2, which is concentrated on the tip cells and their protrusions (Sawamiphak et al., 2010b; Wang et al., 2010b). EphrinB2 is diffusely phosphorylated in the developing retinal vessels, but phosphorylation subsides once the retinal vessels have fully developed (Salvucci et al., 2009; Fig. 6). Expression of a PDZ-mutant EphrinB2 impaired the development of retinal vessels, providing evidence that EphrinB2 signaling is critical to retinal vessel development (Sawamiphak et al., 2010b). In particular, the PDZ-mutant tip cells displayed a significantly reduced ability to form filopodia. This ability of EphrinB2 to promote filopodia extension was confirmed in vitro using primary endothelial cells stimulated with EphB4-Fc (Sawamiphak et al., 2010b) and retinal explants ex vivo (Sawamiphak et al., 2010a).
Fig. 6.
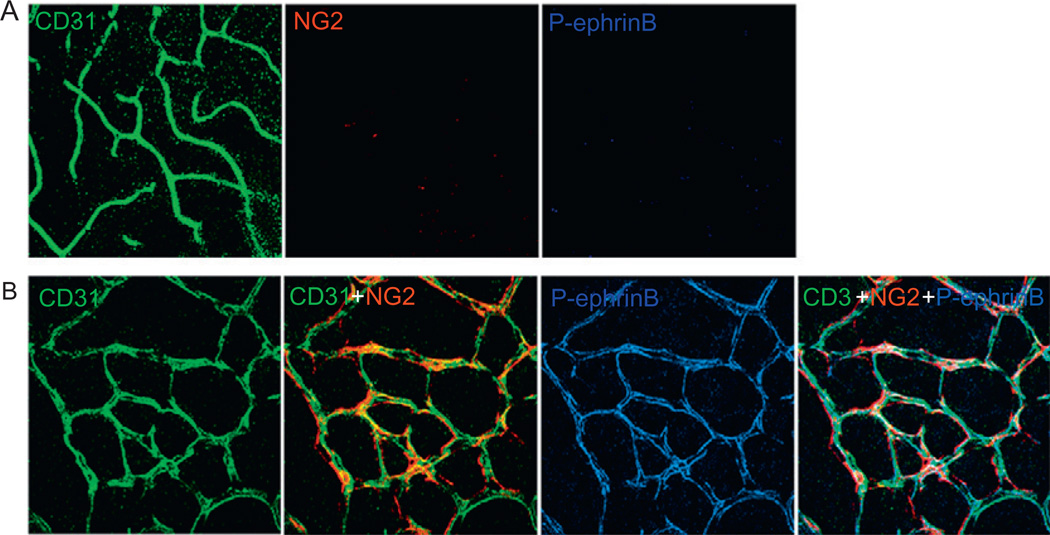
EphrinB is phosphorylated in remodeling but not in fully developed retinal vessels. (A) EphrinB phosphorylation is not detected in fully developed mouse retinal vessels, including the CD31+ endothelial cells and theNG2+ pericytes. (B) EphrinB2 is widely phosphorylated in the remodeling retinal vessels from 6-day-old mice, both in the endothelial cells and in the pericytes.
In the mouse retina, filopodia from adjacent tip cells have been shown to bridge with each other to form connections between developing sprouts, which is an initial step leading to the development of a lumen that permits blood flow in the new vessel (Bentley et al., 2009). The formation of such connecting bridges requires endothelial cell expression of EphrinB and signaling (Sawamiphak et al., 2010b). A similar process of bridging between endothelial cells can be observed in vitro when primary endothelial cells are incubated onto extracellular matrix to form a characteristic network (Salvucci et al., 2006, 2009). Endothelial-to-endothelial cell assembly onto extracellular matrix in vitro is characterized first by the appearance of needle-like extensions and protrusions from endothelial cells resulting in the joining or endothelial cells with each other (Fig. 7A). The appearance of these needle-like protrusions is followed by extensive changes in the cytoskeletal structure and shape of the joined cells, movement of the cell nuclei along the cytoplasm, and the thickening of connecting bridges (Salvucci et al., 2006, 2009). The initial steps in this process of endothelial-to-endothelial cell assembly are associated with time-dependent phosphorylation of EphrinB2 (Salvucci et al., 2009; Fig. 7B). This phosphorylation is likely induced by EphB/EphrinB interactions as specific peptide inhibitors of EphB prevent the formation of cord-like structures on Matrigel (Fig. 7C). A number of experiments provide evidence that such phosphorylation is critical to EphrinB2 function as a regulator of endothelial-to-endothelial cell assembly: if EphrinB2 is silenced in endothelial cells or if the endothelial cells express a signaling-deficient EphrinB2, the assembly of endothelial cells into networks is markedly reduced. Other studies have also shown that if EphrinB2 is overexpressed, appropriate endothelial cell assembly is prevented, providing evidence that an optimal EphrinB2 expression level is required (Salvucci et al., 2009; Sawamiphak et al., 2010b; Wang et al., 2010b).
Fig. 7.
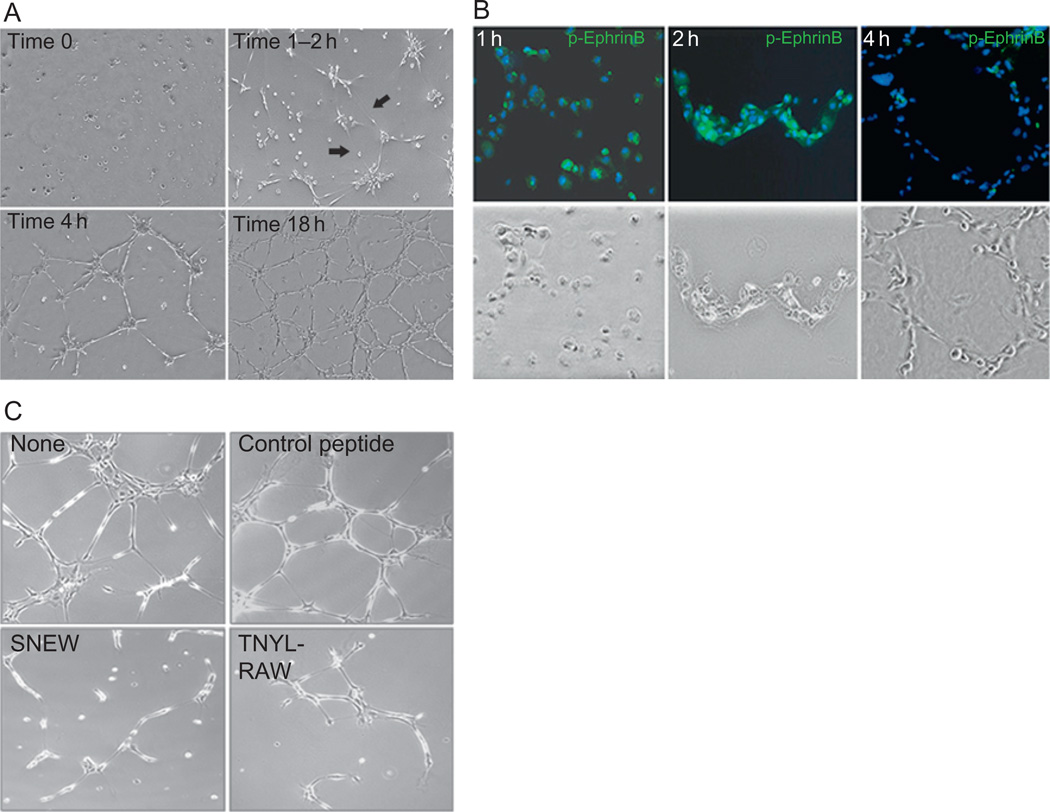
Primary endothelial cells assemble into cord-like structures on extracellular matrix, a process that requires EphB/EphrinB interaction. (A) Time-dependent assembly of primary endothelial cells on Matrigel. Note the appearance after 1–2h of needle-like endothelial cell extensions that eventually form the initial bridging of endothelial cells with each other. (B) EphrinB2 is broadly and time-dependently phosphorylated in endothelial cells as they assemble into cord-like structures. (C) The EphB receptor inhibitors SNEW and TNYL-RAW peptides prevent the assembly of endothelial cells into cord-like structures.
An important question relates to the mechanism by which EphrinB2 is activated in sprouting endothelium, particularly in tip cells where it seems to play an essential role in the path-finding, probing, and extension function of tip cells. One of the possibilities is that, in the retina, astrocytes provide a source of cell-associated EphB4 that stimulates the developing retinal vasculature (Sawamiphak et al., 2010b). Another possibility is that retinal endothelial cells express EphB4, which can stimulate EphrinB2 in interacting endothelial cells (Wang et al., 2010b). Experiments in vitro using primary endothelial cells provide evidence that both EphB4 and EphrinB2 can be simultaneously expressed and that the assembly of vascular networks is dependent upon cell-to-cell interactions resulting in EphrinB signaling induced by endothelial cell-derived EphB4 (Salvucci et al., 2006, 2009). Blocking such EphB/EphrinB cell-to-cell interactions prevents endothelial cell assembly into vascular networks (Salvucci et al., 2006, 2009).
What is the mechanism by which EphrinB2 signaling contributes to sprouting angiogenesis, including “tip” cell function and endothelial cell assembly? Two recent studies in mouse and zebrafish have unveiled a role of EphrinB2 signaling in the regulation of VEGFR2 and VEGFR3 function. If EphrinB2 is silenced or if a signaling-deficient (PDZ-mutant) EphrinB2 is expressed in endothelial cells, VEGFR2 and VEGFR3 signaling is defective, as evidenced by reduced phosphorylation of the receptor following stimulation by the cognate ligand. In addition, activation of the downstream VEGFR signaling components Rac1, Erk1/2, and Akt is reduced (Sawamiphak et al., 2010b; Wang et al., 2010b). Recent studies have concluded that VEGFR trafficking away from the plasma membrane into the endocytic compartment contributes or is necessary for VEGFR function in response to appropriate VEGF ligands (Lampugnani et al., 2006; Lanahan et al., 2010; Sawamiphak et al., 2010b; Wang et al., 2010b). Thus, by promoting the internalization of VEGFR2 and VEGFR3, EphrinB2 signaling may critically regulate VEGFR function in response to the VEGF ligand. It was also observed that EphrinB2 signaling induces some degree of VEGFR2 internalization in the absence of exogenous VEGF (Sawamiphak et al., 2010b). There is evidence for the existence of an endogenous pool of VEGF, which is not secreted outside the cells but contributes to endothelial cell function; it is possible that VEGFR internalized by EphrinB2 signaling is activated by autocrine endogenous VEGF (Lampugnani et al., 2006; Lee et al., 2007).
Besides serving as inducers of EphrinB2 reverse signaling, EphB receptors have also been reported to play a signaling role of their own in endothelial cells. Two studies have concluded that EphB4 forward signaling in endothelial cells represses endothelial cell migration, adhesion, and proliferation in vitro, and may serve to repel endothelial cells from each other and maintain arterial/venous boundaries in capillary beds (Fuller et al., 2003; Kim et al., 2002). Thus, in these studies, EphB4 forward signaling appeared to have opposite effects to those of EphrinB2 reverse signaling in the context of endothelial cell-sprouting angiogenesis. However, other studies have suggested that EphB4 forward signaling in endothelial cells promotes some degree of endothelial cell proliferation and angiogenesis (Stein et al., 1997, 2002). A detailed analysis of EphB2/4 expression and signaling in the context of endothelial cell assembly into cord-like structures in vitro showed characteristic kinetic changes suggestive of a role of EphB forward signaling (Salvucci et al., 2006).When first dispersed on extracellular matrix, primary endothelial cells broadly express surface EphB2/4, but as the cells contact each other to form cord structures, EphB2/4 are internalized and no longer detected on the cell surface (Salvucci et al., 2006). In addition, EphB2/4 become phosphorylated with kinetics suggestive of their activation in association with the occurrence of cell-to-cell contact and receptor internalization (Salvucci et al., 2006). EphB receptor signaling has been linked to the activation of secondary molecules that regulate cytoskeleton structure and cell movement, particularly small Rho family GTPases (Egea and Klein, 2007; Noren and Pasquale, 2004). Consistent with this ability of Eph signaling to regulate cell structure in response to cell-to-cell contact, a typical feature of endothelial cell assembly on extracellular matrix is the time-dependent activation of F (filamentous)-actin filaments in conjunction with cell-to-cell contact and changes in cell shape (Salvucci et al., 2006). The in vivo relevance of these observations remains to be determined, and progress on the role of EphB4 forward signaling in endothelial cell-sprouting angiogenesis will likely benefit from use of mutant mice-expressing signaling-deficient forms of EphB targeted to the endothelial cells.
VI. VESSEL REMODELING AND STABILIZATION
Once formed through sprouting angiogenesis, newly formed vessels undergo a number of changes as a result of the establishment of blood flow, changes in tissue metabolism, endothelial growth factor availability, and other factors. Some of the newly formed vessels regress, whereas others mature through the establishment of a basal membrane composed of extracellular matrix proteins produced by endothelial cells and surrounding stromal cells. A critical step in this process of maturation is the recruitment of pericytes/smooth muscle cells to the nascent vessel, which stabilize the vessel wall and regulate endothelial cell survival, growth, and permeability (Armulik et al., 2005; Hellstrom et al., 2001). Electron microscopy studies have shown that pericytes are inserted into the basal membrane of vessels and come into direct contact with the endothelium through cytoplasmic extensions that penetrate the basement membrane and push deeply onto the endothelial cell surface membrane (Cuevas et al., 1984). Pericytes are found around some blood capillaries, precapillary arterioles, postcapillary venules, and collecting venules, but pericyte coverage of vessels is partial and variable (ranges between 10% and 50%), depending on the tissue. The highest pericyte coverage is found in the central nervous system, where pericytes may regulate the blood–brain barrier functions (Armulik et al., 2010); in the retina, the frequency of pericytes is similar to that of the endothelial cells. Although the derivation of pericytes/mural cells is still somewhat controversial, recent studies suggest that such cells are similar to mesenchymal stem cells, derive from the bone marrow, and display multilineage potential, which explains in part their morphological and phenotypic diversity (Crisan et al., 2008).
Genetic mouse models have implicated several genes in the generation and proper assembly of pericytes, including the PDGF/PDGFR-1, Ang-1/Tie2, and the spingosine 1-phosphate (S1P/S1P1) genes. PDGFB and PDGF-Rβ-null mice die during development with microvascular aneurisms and lack of pericyte coverage on some vessels (Hellstrom et al., 2001). Ang1- or Tie2-null mice die at midgestation with poorly organized basal membranes and reduced coverage/detachment of pericytes (Dumont et al., 1994; Sato et al., 1995; Suri et al., 1996). Conversely, the overexpression of Ang1 promotes pericyte coverage and vessel leakage resistance (Suri et al., 1998; Thurston et al., 1999). Disruption of the s1p1 gene in mice causes prenatal death with vascular abnormalities characterized by defective pericyte coverage (Liu et al., 2000).
EphrinB2 also plays an important role in pericyte function. The mural-cell-specific inactivation of EphrinB2, using PDGF-cre mice, caused the embryos to have edema and extensive hemorrhaging in a variety of tissues and was associated with perinatal death of the mutant mice (Foo et al., 2006). Interestingly, the EphrinB2-null pericytes appeared morphologically normal in many of these mice, but they associated poorly with the vessels, showing a scattered distribution resulting in incomplete vessel coverage (Foo et al., 2006).
During mouse development, EphrinB2 is expressed in the mural cells that cover arteries and veins (Foo et al., 2006). In the adult mouse, EphrinB2 is expressed in a proportion of smooth muscle cells/pericytes surrounding arteries (Shin et al., 2001). Importantly, levels of EphrinB2 expression in smooth muscle cells are comparable to those detected in the arterial endothelium (Shin et al., 2001). Human smooth muscle cells located in the media of adult coronary arteries are partially positive for EphrinB2 (Korff et al., 2008). Human bone marrow-derived mesenchymal stem cells, which either represent pericytes or are related to pericytes, express a functional EphrinB, which can be phosphorylated by EphB4-Fc (Salvucci et al., 2009).
The silencing of EphrinB2 in pericytes/smooth muscle cells derived from the mouse aorta has remarkable effects on cell morphology: the cells become elongated, do not properly spread, and show numerous active lamellipodia protrusions, without evidence of polarization and without cell-to-cell contact (Foo et al., 2006). Some of these defects appeared to be cell-contact-independent, suggesting that EphrinB2 may have cell-autonomous roles (Foo et al., 2006). Functionally, EphrinB2-deficient smooth muscle cells displayed increased, but random, migration, which was associated with defective formation of focal adhesions. Such phenotype was reversed by reexpression of EphrinB2 or activation of Rho-like GTPases (Foo et al., 2006). Consistent with EphrinB2 playing a role in mural cell function, EphB4 (expressed by endothelial cells) induced activation of EphrinB2 on mural cells and enhanced mural/endothelial cell association within tumor blood vessels (Erber et al., 2006).
In vitro, mesenchymal cells/pericytes, which express EphrinB2, characteristically make contact with endothelial cells on extracellular matrix, and when this contact occurs, EphrinB2 phosphorylation is clearly and transiently detected in the mesenchymal stem cells/pericytes (Salvucci et al., 2009). The silencing of EphrinB2 in bone marrow-derived mesenchymal cells/pericytes prevented their proper assembly with endothelial cells (Salvucci et al., 2009; Fig. 8), suggesting that phosphorylation-dependent EphrinB2 signaling is critical for pericytes to make proper contact with endothelial cells (Salvucci et al., 2009).
Fig. 8.
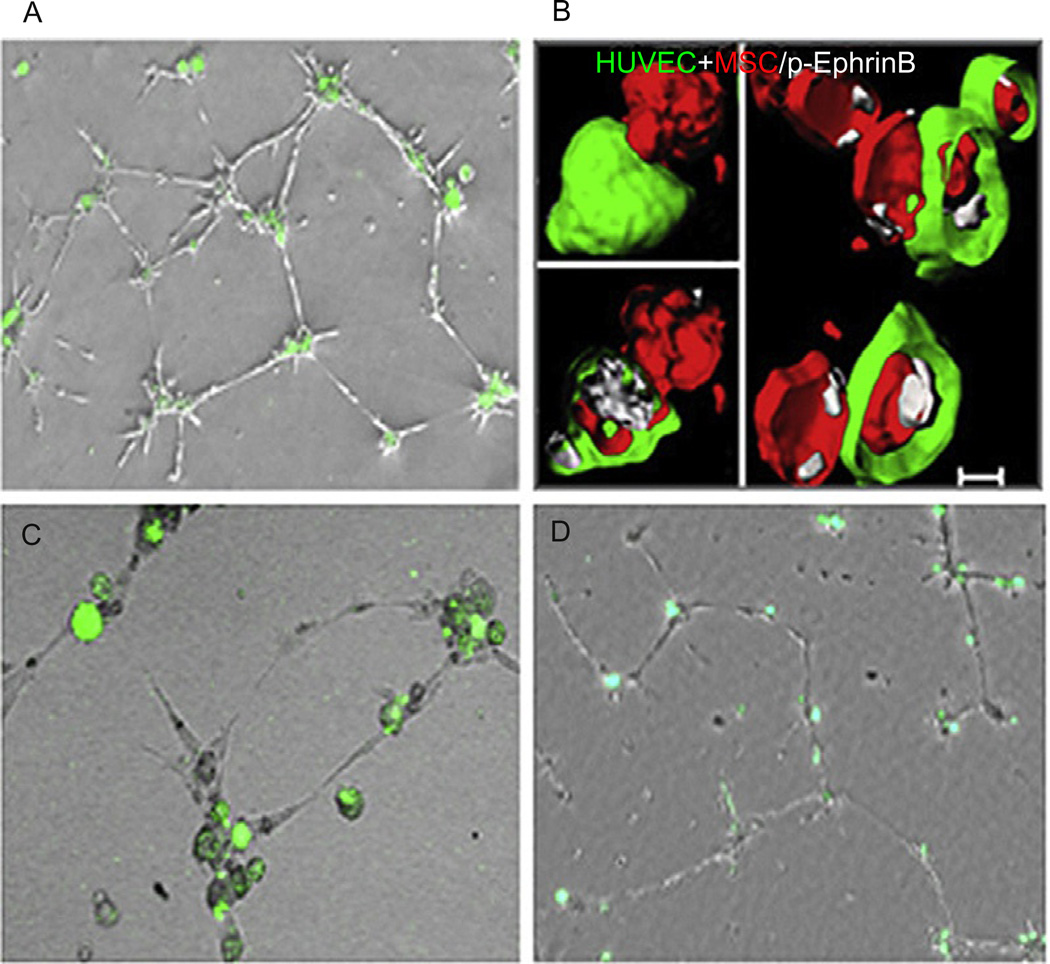
Endothelial cells and mesenchymal stem cells/pericytes assemble in cord-like structures on Matrigel, with mesenchymal stem cells (green) in the nodes anchoring the cord structures. (B) EphrinB is phosphorylated (white) at points of contact between endothelial cells (HUVEC green) and mesenchymal stem cells (MSC red) forming cord-like structures. (C) The silencing of EphrinB2 in mesenchymal stem cells (green) prevents their assembly with endothelial cells (unstained). (D) The silencing of EphrinB2 in endothelial cells (unstained) prevents their assembly with MSC (green).
Little is known about the role of EphB receptors and their signaling in pericytes. Human mesenchymal stem cells express EphB2 and EphB4 (Salvucci et al., 2009), and smooth muscle cells derived from human thymus also express EphB4 (Korff et al., 2008). Activation of EphB4 in smooth muscle cells reduced their ability to migrate, suggesting that EphB4 signaling in pericytes may secure their attachment to the endothelial cells stabilizing the vessel wall (Korff et al., 2008). Vascular smooth muscle cells also express EphA4 receptors, which can be activated by EphrinA1 and by many EphrinB ligands (Ogita et al., 2003). When EphA4 is activated in smooth muscle cells, their ability to form lamellipodia and to spread is inhibited, a process that was linked to inhibition of the small GTPase Rac1 and the kinase Pak1 (Deroanne et al., 2003).
VII. PHYSIOLOGICAL ANGIOGENESIS IN THE ADULT
Physiological angiogenesis in the adult is limited: it only occurs in conjunction with the menstrual cycle and pregnancy, in the skeletal bone during growth, and during wound repair.
The critical role of VEGF as a promoter of angiogenesis during the menstrual cycle, embryo implantation, and pregnancy are well recognized, but little is known about the potential roles of Eph/Ephrins in this context. Studies on corpora lutea have shown that ovulation was followed by a rapid increase in the expression of EphrinB1 and EphrinB2 in the luteinizing granulose (Egawa et al., 2003; Xu et al., 2006) and suggested the occurrence of EphB/EphrinB interactions between the granulosa cells of the corpus luteum and proximal angiogenic capillaries (Egawa et al., 2003; Xu et al., 2006).
Placental implantation of the embryo involves the aggressive invasion of maternal tissues by the embryonic/fetal cytotrophoblasts. This process ultimately results in the cytotrophoblast invasion of the maternal spiral arterioles that supply blood to the placenta, creating a chimeric vasculature composed of maternal and fetal cells (Fisher et al., 1989; Librach et al., 1991). The invasive behavior of the embryonic/fetal cytotrophoblasts is accompanied by remarkable changes in gene expression and phenotype; in particular, they acquire vascular-type markers (Damsky et al., 1992; Zhou et al., 1997) and produce proangiogenic factors (Zhou et al., 2003). Important changes occurring in the cytotrophoblasts as they penetrate the uterine wall are the loss of EphB4 expression and an increase in EphrinB2 expression.Notch signaling is a critical regulator of the divergent expression of EphrinB2 and EphB4 in these cells (Hunkapiller et al., 2011; Red-Horse et al., 2005). This phenotypic change in embryonic/fetal cytotrophoblasts has been proposed to underlie the selective tropism of the cytotrophoblasts toward the maternal arterioles, thus limiting interactions with the maternal venules (Red-Horse et al., 2005).
Angiogenesis in the context of skin wound healing occurs through endothelial cell sprouting from capillaries at the edge of the wound when the dermis is injured. The process is driven by VEGF-A (Bao et al., 2009) that is first produced by platelets, which contribute to the formation of the blood clot that initially seals the wound. Inflammatory monocytes and neutrophils are additional sources of VEGF in the wound and represent a local defense system against pathogens. They are recruited by a variety of signals, including complement components, platelet degranulation products, and potentially bacterial particles (Banks et al., 1998; Mohle et al., 1997). Sprouting wound vessels are components of the “granulation tissue,” which is a hallmark of wounds undergoing repair. Besides sprouting vessels, the granulation tissue contains a fibrin clot, fibroblasts, collagen, and inflammatory cells (Bao et al., 2009; Murohara et al., 1998). Granulation tissue undergoes extensive remodeling in repairing wounds, and the process is orchestrated by a series of enzymes including MMP1, MMP2, urokinase-type, and tissue-type plasminogen activators, and plasminogen activator inhibitor (Schultz and Wysocki, 2009).
A study reported that circulating mouse neutrophils express EphrinA2 and B2 (Schruefer et al., 2006). Other studies have demonstrated that inflammatory macrophages express EphrinB2 and suggested a role for monocytederived EphrinB2 in the stimulation of venous endothelium that expresses EphB4 (Yuan et al., 2000, 2004). Recent studies have confirmed the expression of EphrinB2 in the developing dermal vasculature of mice (Wang et al., 2010b). In a murine model of wound healing in the skin, the sprouting endothelial cells from the margins of the wound expressed EphrinB2, which was prominently and time-dependently phosphorylated in these cells, indicative of active signaling (Salvucci et al., 2009; Fig. 9A). In contrast, EphrinB2 was not phosphorylated in the surrounding normal vessels or in the new capillaries once sprouting had subsided and the granulation tissue was well developed (Salvucci et al., 2009). Additionally, EphrinB2 expression and phosphorylation were transiently detected in wound-infiltrating inflammatory cells (Salvucci et al., 2009).
Fig. 9.
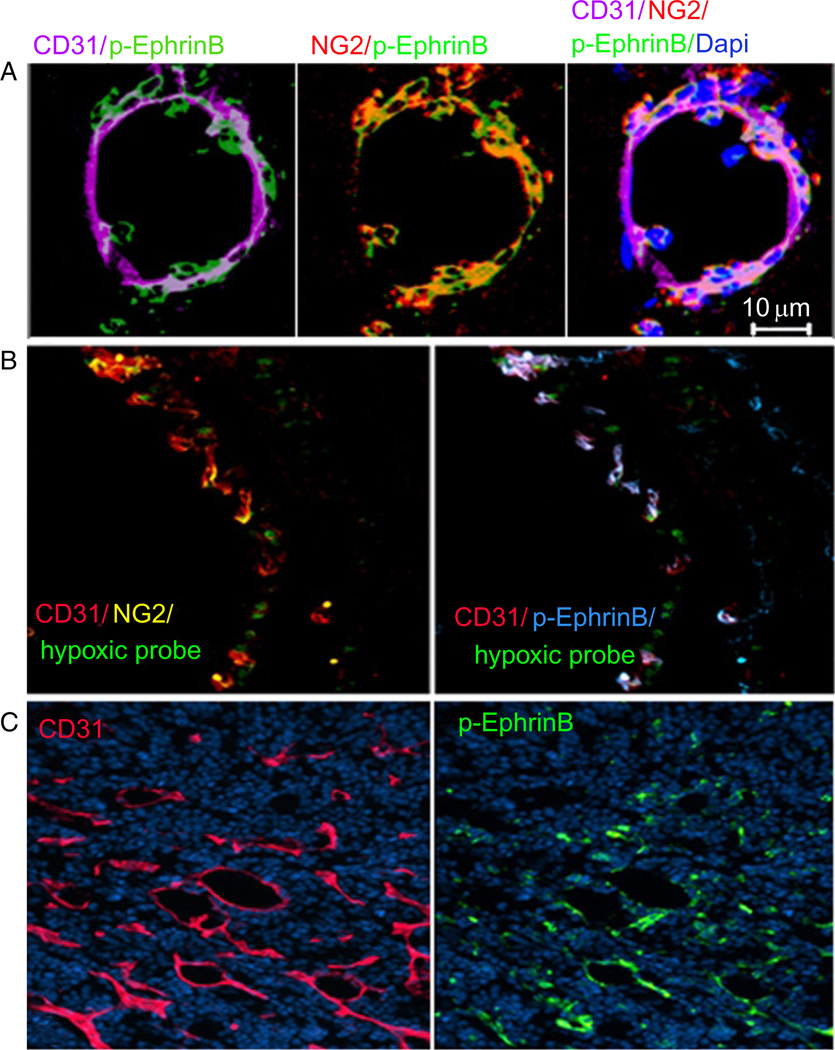
(A) EphrinB is phosphorylated in angiogenic vessels associated with skin wound healing. (B) EphrinB is phosphorylated in angiogenic retinal vessels induced by hypoxia (areas of hypoxia visualized in green; ROP model). (C) EphrinB is phosphorylated in angiogenic tumor vessels. CD31 identifies endothelial cells; NG2 pericytes/mural cells.
VIII. PATHOLOGICAL ANGIOGENESIS
A. Neovascular Disorders of the Eye
Intraocular neovascularization is a pathological complication of many eye diseases, including diabetic retinopathy, retinopathy of prematurity, and age-related (neovascular, wet-type) macular degeneration (AMD). Since these conditions represent the major cause for blindness, control of pathological angiogenesis is an important therapeutic goal. Retinal ischemia and increased VEGF expression are often the principal causes of pathological retinal angiogenesis (Alon et al., 1995; Fraisl et al., 2009; Miller et al., 1994). Oxygen levels and the levels of VEGF are tightly regulated in the normal retina (Stenzel et al., 2011). Hypoxia regulates expression of VEGF largely through the activities of the transcription factors HIFs (hypoxia inducing factors) (Semenza, 2003; Tang et al., 2011). The HIF-1α subunit is maintained at low levels during normoxia through an oxygen-dependent degradation process, which is mediated by the von Hippel Lindau (VHL) protein, a component of the E3 ubiquitin ligase complex (Semenza, 2011). Once the oxygen pressure drops below 5%, HIF-1α is not sufficiently degraded, resulting in the activation of genes involved in the maintenance of oxygen homeostatic levels. One of these target genes is VEGF, which promotes the growth of blood vessels toward hypoxic zones (Ferrara et al., 2003; Gerhardt et al., 2003). Consistent with its role in the degradation of HIF-1α, loss-of-function mutations of VHL result in increased tumor angiogenesis (Pugh and Ratcliffe, 2003).
Abnormally high levels of VEGF and pathological angiogenesis also characterize the wet-type AMD (Jager et al., 2008). In this condition, choroidal neovascularization causes exudation and hemorrhaging, which impair the central portion of the retina that is responsible for high-resolution vision (Jager et al., 2008). VEGF is detected at high levels in the choroidal membranes from patients with wet AMD (Kvanta et al., 1996; Lopez et al., 1996); various approaches to limit VEGF activity in the eye have demonstrated a marked clinical benefit to the patients.
A number of mouse models of ischemia-induced intraocular neovascularization have been developed, including the retinopathy of immaturity (ROP) model, which has been useful to study ischemia-induced retinal angiogenesis (Smith et al., 1994). In this model, 7-day-old mice are exposed to high (75%) oxygen tension for a period of 5 days and then are exposed to room air. High oxygen tension causes physiological postnatal retinal angiogenesis to come to a stop and promotes degeneration of the retinal vessels that had recently sprouted. Once the retinas are reexposed to air oxygen, retinal neovascularization resumes with vigorous retinal vessel sprouting after 3 days (Smith et al., 1994). These sprouting retinal vessels express EphrinB2, which is phosphorylated (Salvucci et al., 2009), indicative of activity (Fig. 9B). Once the sprouting subsides in the retinal vessels, EphrinB2 phosphorylation also subsides, without change in the levels of EphrinB2 expression (Salvucci et al., 2009). These results show that hyperoxia-induced retinal neovascularization is associated with EphrinB2 activation and thus is similar in this respect to the occurrence of EphrinB2 phosphorylation during physiological postnatal angiogenesis of the retina.
B. Tumor Angiogenesis
Angiogenesis, a hallmark of a growing malignancy, often starts when the tumors are quite small in size (1–2mm) and is principally driven by VEGF-A produced by the tumor cells and by cells in the tumor microenvironment. Tumor hypoxia is believed to represent an important inducer of tumor-associated VEGF (Dayan et al., 2008; Ferrara and Kerbel, 2005; Mantovani et al., 2002; Murdoch et al., 2008), which is an indispensable driver of tumor angiogenesis in preclinical mouse models (Ferrara and Kerbel, 2005; Kim et al., 1993). Tumor angiogenesis resembles physiological angiogenesis but presents notable differences (Chung et al., 2011). A principal difference is that tumor angiogenesis is not self-limited but appears to be a self-perpetuating and persistent process that is not turned off once vessels are formed. Tumor vessels are disorganized compared to normal vessels and display a number of abnormalities, including fragility, reduced number of pericytes, and leakiness with a propensity to produce exudates and to bleed. These abnormalities have been linked to excess VEGF-A, and thus VEGF neutralization has been reported to somewhat “normalize” the tumor vasculature (Jain, 2005). The tumor cells, particularly melanomas and glioblastomas, may undergo a process of transformation, named “vasculogenic mimicry,” such that the tumor cells or the differentiated tumor stem cells acquire phenotypic characteristics of endothelial cells and become components of the vessel wall (Hendrix et al., 2003a,b; Ricci-Vitiani et al., 2010; Wang et al., 2010a).
A number of preclinical and clinical studies using inhibitors of VEGF have identified VEGF-independent pathways of tumor angiogenesis, which may account for the poor responses and/or acquired resistance to VEGF neutralization (Shojaei et al., 2007). One such pathway involves the neutrophilderived proangiogenic factor Bv8 (also known as prokineticin 2), which drives endothelial cell proliferation and migration in the absence of VEGF-A (Shojaei et al., 2007, 2009). Other pathways markedly modulate VEGF expression and/or VEGF-A-induced responses, both physiologically and in cancer, including the Dll4/Notch (Hellstrom et al., 2007; Noguera-Troise et al., 2006; Ridgway et al., 2006), Angiopoietin-1/Tie2 and Angiopoietin-2/Tie2 (Augustin et al., 2009), platelet-derived growth factors (PDGFs)/PDGF-receptor β (Bergers et al., 2003), and TGFβ1/TGFβ receptor II (Chung et al., 2011; Fridlender et al., 2009). The Eph/Ephrin signaling pathways can also regulate VEGF/VEGFR signaling in the context of cancer, but the system is complex (Bruckner et al., 1997; Pasquale, 2010; Sawamiphak et al., 2010a,b).
The functions of the Eph/Ephrin system in cancer are complex due to the fact that many tumor cells express Eph receptors and ligands, albeit to varying degrees (Davalos et al., 2006; Huang et al., 2007; Noren and Pasquale, 2007; Zhuang et al., 2010), which can stimulate or be stimulated by Eph/Ephrin molecules in the tumor endothelium and in other cells of the tumor microenvironment. Thus, the outcome of Eph receptor and ligand signaling in the tumor cells differs in different tumors (no effect, as opposed to inhibition or stimulation of tumor growth) (Batlle et al., 2002; Dohn et al., 2001; Dopeso et al., 2009; Jin et al., 2006; Kumar et al., 2009; Miao et al., 2009; Noren and Pasquale, 2007; Noren et al., 2006; Ruhe et al., 2007; Smith et al., 2004; Yu et al., 1999).
There are a few situations that best illustrate the complex roles of Eph/Ephrin in tumor growth. For example, a number of studies have provided evidence that EphB2/EphB4 signaling in certain tumor cells suppresses tumor growth or promotes tumor dormancy. In particular, it was reported that soluble EphrinB-Fc reduced colon and breast cancer cell growth in vitro and tumor progression in mice by pharmacologically activating tumor cell-associated EphB4 (Batlle et al., 2002; Noren and Pasquale, 2007; Noren et al., 2006). Consistent with a tumor-suppressor role of EphB4 signaling, a dominant-negative form of EphB4 promoted colorectal tumor growth (Dopeso et al., 2009). Also, consistent with the notion that EphB2 and EphB4 act as inhibitors of tumor growth, EphB2 and EphB4 are mostly inactive in tumors as judged by low levels of phosphorylation in many malignant cells and are expressed at higher levels in benign tumors of the same lineage (Kumar et al., 2009; Miao et al., 2005; Noren and Pasquale, 2007; Noren et al., 2006). Thus, it was proposed that EphB signaling in the tumor cells may inhibit oncogenic signaling pathways operating in the tumor cells thereby reducing tumor growth, may promote repulsive interactions between the EphB-expressing tumor cells and the EphrinB1-expressing normal tissue that would restrict tumor cell invasion, or may promote tumor cell adherence to each other preventing local tissue infiltration (Cortina et al., 2007; Kemp et al., 2009; Pasquale, 2010).
In contrast with these results, other studies have indicated that EphB4 signaling promotes tumor growth, perhaps due to a loss of tumor-inhibitory function or to acquisition of ligand-independent activities (Pasquale, 2010). In some studies, activation of tumor-associated EphB4 enhanced tumor growth, motility, and metastasis of prostate, bladder, colorectal, and melanoma cancer cells (Xia et al., 2005, 2006; Yang et al., 2006). Also, knockdown of EphB4 in colorectal and ovarian cancer cells inhibited tumor growth and metastasis (Kumar et al., 2007, 2009). Tumor-associated expression of EphB4 stimulated tumor angiogenesis and tumor growth by activating EphrinB2 on the tumor endothelium (Noren et al., 2006), and overexpression of EphrinB2 in ovarian and melanoma tumor cells correlated with increased tumor invasion and poor prognosis, perhaps by promoting EphB4 activation in the tumor cells (Castellvi et al., 2006; Meyer et al., 2005). Deregulated expression of EphB4 and EphrinB2 in KSHV (Kaposi’s sarcoma-associated herpes virus)-infected KS cells is also believed to contribute to tumor growth (Masood et al., 2005).
The complexities of Eph/Ephrin interactions in growing tumors and the difficulties in generalizing their function in cancer suggest that careful experimentation with controlled systems may be useful. In line with this, a simplified tumor model unequivocally showed that EphrinB2 signaling in tumor-associated vessels promotes tumor angiogenesis, much alike the situation in physiological angiogenesis (Sawamiphak et al., 2010b). Using mouse tumor models of intracranial gliomas and subcutaneous astrocytoma, the authors showed that tumor growth and tumor angiogenesis were significantly reduced in EphrinB2-deficient tumor-bearing mice in comparison to wild-type control mice (Sawamiphak et al., 2010b). The interpretation of these results is that sprouting angiogenesis in tumors resembles physiological angiogenesis and as such is similarly regulated by EphrinB2 signaling via modulation of VEGF-A/VEGFR2 function (Sawamiphak et al., 2010b; Wang et al., 2010b). Consistent with EphrinB signaling playing an important role in tumor angiogenesis, the angiogenic tumor vessels in experimental MOPC315 tumors displayed an intensely phosphorylated EphrinB, particularly at the tumor margins where angiogenesis was most prominent (Salvucci et al., 2009; Fig. 9C). In addition, tumors that express EphB4 promote angiogenesis through interaction with EphrinB2 expressed by the tumor endothelium (Noren et al., 2006).
IX. THERAPEUTIC IMPLICATIONS
Pathological angiogenesis in the adult is mostly associated with neovascular diseases of the eye, including wet macular degeneration and diabetic retinopathy, and with tumor angiogenesis, which contributes to tumor growth in many types of cancer. It is now clear that EphrinB2 signaling plays a critical role in promoting VEGF-induced endothelial cell sprouting and orchestrating endothelial-to-endothelial and endothelial-to-pericyte assembly. For these reasons, EphrinB2 and its ligands are desirable therapeutic targets for antiangiogenic therapy. However, the complexities of cancer, where Eph/Ephrin interactions involve not only the tumor vasculature but also the tumor cells and other cells resulting in strikingly different tumor outcomes, are a challenge for the development of Eph/Ephrin-based cancer drugs. Thus, potential application of Eph/Ephrin targeting to cancer treatment will need additional study and validation for individual cancer types, stage, and other variables.
A number of approaches have demonstrated to effectively inhibit Eph/Ephrin interactions. Monomeric soluble EphB4 ectodomain, which inhibits EphB4 forward signaling and EphrinB2 reverse signaling, has been shown to reduce angiogenesis in vivo and experimental tumor growth in mice (Kertesz et al., 2006; Scehnet et al., 2009). EphB4 agonist antibodies could be useful in the context of selected EphB4-expressing tumors as they could promote EphB4 tumor-suppressor activity and at the same time inhibit EphrinB2-induced tumor angiogenesis (Krasnoperov et al., 2010; Noren and Pasquale, 2004; Noren et al., 2006). Specific antibodies to EphB2 have been generated that can effectively block interaction with Ephrins and kill EphB2-expressing tumor cells when conjugated with the cytotoxic drug auristatin (Mao et al., 2004; Xu et al., 2009). Phage display allowed the identification of specific peptides that bind EphB2 and EphB4 at nanomolar concentrations and can readily inhibit endothelial-to-endothelial cell assembly in vitro and experimental angiogenesis in vivo (Chrencik et al., 2006, 2007; Koolpe et al., 2005; Salvucci et al., 2006, 2009). A neutralizing antibody to EphrinB2 was reported to reduce vessel number, but not size, in an experimental model of human glioblastoma (Li et al., 2011).
X. CONCLUSIONS
In this review, we have discussed evidence for a role of the B family of Ephs and Ephrins in angiogenesis. We have described how B Ephs and Ephrins signaling play critical roles in developmental and postnatal angiogenesis in physiology and disease. Thus, B Ephs and Ephrins are promising targets to modulate angiogenesis. Several approaches to block EphB/EphrinB function seem to be very effective at reducing angiogenesis in experimental models. However, many complexities of EphB and EphrinB signaling are not understood, particularly how they integrate with other signaling pathways. The context-dependent functions of B Ephs and Ephrins in cancer are poorly understood and may require a better understanding of the role of Eph/Ephrin in cell interactions between tumor cells and the tumor microenvironment as well as understanding how EphB/EphrinB signaling integrates with oncogenic signaling pathways. Research in the coming years will likely decipher the diverse functions of Ephs and Ephrins in the context of cancer. This will be an important advance that may open a wide range of therapeutic opportunities.
ACKNOWLEDGMENTS
This work was supported by the Intramural Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892. The authors wish to thank Dr. Douglas Lowy for his support and Inn Inn Chen for reviewing the manuscript.
REFERENCES
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels has implications for retinopathy of prematurity. Nat. Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis homeostasis through the angiopoietin–tie system. Nat. Rev. Mol. Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements cancer biology. Br. J. Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The Notch ligands Dll4 Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Bentley K, Mariggi G, Gerhardt H, Bates PA. Tipping the balance: robustness of tip cell selection, migration fusion in angiogenesis. PLoS Comput. Biol. 2009;5:e1000549. doi: 10.1371/journal.pcbi.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bong YS, Lee HS, Carim-Todd L, Mood K, Nishanian TG, Tessarollo L, Daar IO. ephrinB1 signals from the cell surface to the nucleus by recruitment of STAT3. Proc. Natl. Acad. Sci. U.S.A. 2007;104:17305–17310. doi: 10.1073/pnas.0702337104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech. Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Brantley-Sieders D, Schmidt S, Parker M, Chen J. Eph receptor tyrosine kinases in tumor tumor microenvironment. Curr. Pharm. Des. 2004;10:3431–3442. doi: 10.2174/1381612043383160. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pasquale EB, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- Castellvi J, Garcia A, de la Torre J, Hernandez J, Gil A, Xercavins J, Ramon y Cajal S. Ephrin B expression in epithelial ovarian neoplasms correlates with tumor differentiation angiogenesis. Hum. Pathol. 2006;37:883–889. doi: 10.1016/j.humpath.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev. Cell. 2009;17:377–386. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong LD, Park EK, Latimer E, Friesel R, Daar IO. Fibroblast growth factor receptor-mediated rescue of x-ephrin B1-induced cell dissociation in Xenopus embryos. Mol. Cell. Biol. 2000;20:724–734. doi: 10.1128/mcb.20.2.724-734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrencik JE, Brooun A, Recht MI, Kraus ML, Koolpe M, Kolatkar AR, Bruce RH, Martiny-Baron G, Widmer H, Pasquale EB, Kuhn P. Structure thermodynamic characterization of the EphB4/Ephrin-B2 antagonist peptide complex reveals the determinants for receptor specificity. Structure. 2006;14:321–330. doi: 10.1016/j.str.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Chrencik JE, Brooun A, Recht MI, Nicola G, Davis LK, Abagyan R, Widmer H, Pasquale EB, Kuhn P. Three-dimensional structure of the EphB2 receptor in complex with an antagonistic peptide reveals a novel mode of inhibition. J. Biol. Chem. 2007;282:36505–36513. doi: 10.1074/jbc.M706340200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat. Rev. Cancer. 2011;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- Cortina C, Palomo-Ponce S, Iglesias M, Fernandez-Masip JL, Vivancos A, Whissell G, Huma M, Peiro N, Gallego L, Jonkheer S, Davy A, Lloreta J, et al. EphB– ephrin-B interactions suppress colorectal cancer progression by compartmentalizing tumor cells. Nat. Genet. 2007;39:1376–1383. doi: 10.1038/ng.2007.11. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Henkemeyer M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature. 2001;413:174–179. doi: 10.1038/35093123. [DOI] [PubMed] [Google Scholar]
- Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin-B2 reverse signaling is required for axon pathfinding cardiac valve formation but not early vascular development. Dev. Biol. 2004;271:263–271. doi: 10.1016/j.ydbio.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Cowan CW, Shao YR, Sahin M, Shamah SM, Lin MZ, Greer PL, Gao S, Griffith EC, Brugge JS, Greenberg ME. Vav family GEFs link activated Ephs to endocytosis axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Cuevas P, Gutierrez-Diaz JA, Reimers D, Dujovny M, Diaz FG, Ausman JI. Pericyte endothelial gap junctions in human cerebral capillaries. Anat. Embryol. (Berl) 1984;170:155–159. doi: 10.1007/BF00319000. [DOI] [PubMed] [Google Scholar]
- Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J. Clin. Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos V, Dopeso H, Castano J, Wilson AJ, Vilardell F, Romero-Gimenez J, Espin E, Armengol M, Capella G, Mariadason JM, Aaltonen LA, Schwartz S, Jr, et al. EPHB4 survival of colorectal cancer patients. Cancer Res. 2006;66:8943–8948. doi: 10.1158/0008-5472.CAN-05-4640. [DOI] [PubMed] [Google Scholar]
- Dayan F, Mazure NM, Brahimi-Horn MC, Pouyssegur J. A dialogue between the hypoxia-inducible factor the tumor microenvironment. Cancer Microenviron. 2008;1:53–68. doi: 10.1007/s12307-008-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroanne C, Vouret-Craviari V, Wang B, Pouyssegur J. EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J. Cell Sci. 2003;116:1367–1376. doi: 10.1242/jcs.00308. [DOI] [PubMed] [Google Scholar]
- Dohn M, Jiang J, Chen X. Receptor tyrosine kinase EphA2 is regulated by p53- family proteins induces apoptosis. Oncogene. 2001;20:6503–6515. doi: 10.1038/sj.onc.1204816. [DOI] [PubMed] [Google Scholar]
- Dopeso H, Mateo-Lozano S, Mazzolini R, Rodrigues P, Lagares-Tena L, Ceron J, Romero J, Esteves M, Landolfi S, Hernandez-Losa J, Castano J, Wilson AJ, et al. The receptor tyrosine kinase EPHB4 has tumor suppressor activities in intestinal tumorigenesis. Cancer Res. 2009;69:7430–7438. doi: 10.1158/0008-5472.CAN-09-0706. [DOI] [PubMed] [Google Scholar]
- Dravis C, Henkemeyer M. Ephrin-B reverse signaling controls septation events at the embryonic midline through separate tyrosine phosphorylation-independent signaling avenues. Dev. Biol. 2011;355:138–151. doi: 10.1016/j.ydbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Egawa M, Yoshioka S, Higuchi T, Sato Y, Tatsumi K, Fujiwara H, Fujii S. Ephrin B1 is expressed on human luteinizing granulosa cells in corpora lutea of the early luteal phase: the possible involvement of the B class Eph–ephrin system during corpus luteum formation. J. Clin. Endocrinol. Metab. 2003;88:4384–4392. doi: 10.1210/jc.2002-021910. [DOI] [PubMed] [Google Scholar]
- Egea J, Klein R. Bidirectional Eph–ephrin signaling during axon guidance. Trends Cell Biol. 2007;17:230–238. doi: 10.1016/j.tcb.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Erber R, Eichelsbacher U, Powajbo V, Korn T, Djonov V, Lin J, Hammes HP, Grobholz R, Ullrich A, Vajkoczy P. EphB4 controls blood vascular morphogenesis during postnatal angiogenesis. EMBO J. 2006;25:628–641. doi: 10.1038/sj.emboj.7600949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fisher SJ, Cui TY, Zhang L, Hartman L, Grahl K, Zhang GY, Tarpey J, Damsky CH. Adhesive degradative properties of human placental cytotrophoblast cells in vitro. J. Cell Biol. 1989;109:891–902. doi: 10.1083/jcb.109.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen metabolism. Dev. Cell. 2009;16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukai J, Yokote H, Yamanaka R, Arao T, Nishio K, Itakura T. EphA4 promotes cell proliferation migration through a novel EphA4-FGFR1 signaling pathway in the human glioma U251 cell line. Mol. Cancer Ther. 2008;7:2768–2778. doi: 10.1158/1535-7163.MCT-07-2263. [DOI] [PubMed] [Google Scholar]
- Fuller T, Korff T, Kilian A, Dandekar G, Augustin HG. Forward EphB4 signaling in endothelial cells controls cellular repulsion segregation from ephrinB2 positive cells. J. Cell Sci. 2003;116:2461–2470. doi: 10.1242/jcs.00426. [DOI] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, Yancopoulos GD. Ephrin-B2 selectively marks arterial vessels neovascularization sites in the adult, with expression in both endothelial smooth-muscle cells. Dev. Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- Gariano RF, Gardner TW. Retinal angiogenesis in development disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation signaling. EMBO J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol. Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia abnormal vascular morphogenesis. J. Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Molecular plasticity of human melanoma cells. Oncogene. 2003a;22:3070–3075. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry tumour-cell plasticity: lessons from melanoma. Nat. Rev. Cancer. 2003b;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himanen JP, Nikolov DB. Eph signaling: a structural view. Trends Neurosci. 2003;26:46–51. doi: 10.1016/s0166-2236(02)00005-x. [DOI] [PubMed] [Google Scholar]
- Himanen JP, Rajashankar KR, Lackmann M, Cowan CA, Henkemeyer M, Nikolov DB. Crystal structure of an Eph receptor–ephrin complex. Nature. 2001;414:933–938. doi: 10.1038/414933a. [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Gish GD, Roth RA, Songyang Z, Cantley LC, Henkemeyer M, Yancopoulos GD, Pawson T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997;16:3877–3888. doi: 10.1093/emboj/16.13.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Reeves K, Han X, Fairchild C, Platero S, Wong TW, Lee F, Shaw P, Clark E. Identification of candidate molecular markers predicting sensitivity in solid tumors to dasatinib: rationale for patient selection. Cancer Res. 2007;67:2226–2238. doi: 10.1158/0008-5472.CAN-06-3633. [DOI] [PubMed] [Google Scholar]
- Hunkapiller NM, Gasperowicz M, Kapidzic M, Plaks V, Maltepe E, Kitajewski J, Cross JC, Fisher SJ. A role for Notch signaling in trophoblast endovascular invasion in the pathogenesis of pre-eclampsia. Development. 2011;138:2987–2998. doi: 10.1242/dev.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh-Do U, Vindis C, Liu H, Cerretti DP, McGrew JT, Enriquez M, Chen J, Daniel TO. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment angiogenesis. J. Cell Sci. 2002;115:3073–3081. doi: 10.1242/jcs.115.15.3073. [DOI] [PubMed] [Google Scholar]
- Irie F, Okuno M, Pasquale EB, Yamaguchi Y. EphrinB-EphB signalling regulates clathrin-mediated endocytosis through tyrosine phosphorylation of synaptojanin 1. Nat. Cell Biol. 2005;7:501–509. doi: 10.1038/ncb1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N. Engl. J. Med. 2008;358:2606–2617. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- Jin YJ, Wang J, Qiao C, Hei TK, Brandt-Rauf PW, Yin Y. Anovel mechanism for p53 to regulate its target gene ECK in signaling apoptosis. Mol. Cancer Res. 2006;4:769–778. doi: 10.1158/1541-7786.MCR-06-0178. [DOI] [PubMed] [Google Scholar]
- Kalo MS, Pasquale EB. Signal transfer by Eph receptors. Cell Tissue Res. 1999;298:1–9. [PubMed] [Google Scholar]
- Kemp HA, Cooke JE, Moens CB. EphA4 EfnB2a maintain rhombomere coherence by independently regulating intercalation of progenitor cells in the zebrafish neural keel. Dev. Biol. 2009;327:313–326. doi: 10.1016/j.ydbio.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz N, Krasnoperov V, Reddy R, Leshanski L, Kumar SR, Zozulya S, Gill PS. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4- EphrinB2 interaction, modulates angiogenesis, inhibits tumor growth. Blood. 2006;107:2330–2338. doi: 10.1182/blood-2005-04-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Kim I, Ryu YS, Kwak HJ, Ahn SY, Oh JL, Yancopoulos GD, Gale NW, Koh GY. EphB ligand, ephrinB2, suppresses the VEGF- angiopoietin 1-induced Ras/mitogen-activated protein kinase pathway in venous endothelial cells. FASEB J. 2002;16:1126–1128. doi: 10.1096/fj.01-0805fje. [DOI] [PubMed] [Google Scholar]
- Klein R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat. Neurosci. 2009;12:15–20. doi: 10.1038/nn.2231. [DOI] [PubMed] [Google Scholar]
- Knoll B, Drescher U. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J. Neurosci. 2004;24:6248–6257. doi: 10.1523/JNEUROSCI.0985-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolpe M, Burgess R, Dail M, Pasquale EB. EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J. Biol. Chem. 2005;280:17301–17311. doi: 10.1074/jbc.M500363200. [DOI] [PubMed] [Google Scholar]
- Korff T, Braun J, Pfaff D, Augustin HG, Hecker M. Role of ephrinB2 expression in endothelial cells during arteriogenesis: impact on smooth muscle cell migration monocyte recruitment. Blood. 2008;112:73–81. doi: 10.1182/blood-2007-12-128835. [DOI] [PubMed] [Google Scholar]
- Krasnoperov V, Kumar SR, Ley E, Li X, Scehnet J, Liu R, Zozulya S, Gill PS. Novel EphB4 monoclonal antibodies modulate angiogenesis inhibit tumor growth. Am. J. Pathol. 2010;176:2029–2038. doi: 10.2353/ajpath.2010.090755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms functions of Eph ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- Kumar SR, Masood R, Spannuth WA, Singh J, Scehnet J, Kleiber G, Jennings N, Deavers M, Krasnoperov V, Dubeau L, Weaver FA, Sood AK, et al. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals predicts poor outcome. Br. J. Cancer. 2007;96:1083–1091. doi: 10.1038/sj.bjc.6603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SR, Scehnet JS, Ley EJ, Singh J, Krasnoperov V, Liu R, Manchanda PK, Ladner RD, Hawes D, Weaver FA, Beart RW, Singh G, et al. Preferential induction of EphB4 over EphB2 its implication in colorectal cancer progression. Cancer Res. 2009;69:3736–3745. doi: 10.1158/0008-5472.CAN-08-3232. [DOI] [PubMed] [Google Scholar]
- Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest. Ophthalmol. Vis. Sci. 1996;37:1929–1934. [PubMed] [Google Scholar]
- Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization signaling from intracellular compartments. J. Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan AA, Hermans K, Claes F, Kerley-Hamilton JS, Zhuang ZW, Giordano FJ, Carmeliet P, Simons M. VEGF receptor 2 endocytic trafficking regulates arterial morphogenesis. Dev. Cell. 2010;18:713–724. doi: 10.1016/j.devcel.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach J, Klein R. Release of full-length EphB2 receptors from hippocampal neurons to cocultured glial cells. J. Neurosci. 2006;26:11575–11581. doi: 10.1523/JNEUROSCI.2697-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Bong YS, Moore KB, Soria K, Moody SA, Daar IO. Dishevelled mediates ephrinB1 signalling in the eye field through the planar cell polarity pathway. Nat. Cell Biol. 2006;8:55–63. doi: 10.1038/ncb1344. [DOI] [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Mood K, Battu G, Ji YJ, Singh A, Daar IO. Fibroblast growth factor receptor-induced phosphorylation of ephrinB1 modulates its interaction with Dishevelled. Mol. Biol. Cell. 2009;20:124–133. doi: 10.1091/mbc.E08-06-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JL, Sainson RC, Oon CE, Turley H, Leek R, Sheldon H, Bridges E, Shi W, Snell C, Bowden ET, Wu H, Chowdhury PS, et al. DLL4-Notch signaling mediates tumor resistance to anti-VEGF therapy in vivo. Cancer Res. 2011;71:6073–6083. doi: 10.1158/0008-5472.CAN-11-1704. [DOI] [PubMed] [Google Scholar]
- Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J. Cell Biol. 1991;113:437–449. doi: 10.1083/jcb.113.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Gish GD, Songyang Z, Pawson T. The carboxyl terminus of B class ephrins constitutes a PDZ domain binding motif. J. Biol. Chem. 1999;274:3726–3733. doi: 10.1074/jbc.274.6.3726. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, et al. Edg-1, the G proteincoupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc. Natl. Acad. Sci. U.S.A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidal neovascular membranes. Invest. Ophthalmol. Vis. Sci. 1996;37:855–868. [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Maekawa H, Oike Y, Kanda S, Ito Y, Yamada Y, Kurihara H, Nagai R, Suda T. Ephrin-B2 induces migration of endothelial cells through the phosphatidylinositol- kinase pathway promotes angiogenesis in adult vasculature. Arterioscler. Thromb. Vasc. Biol. 2003;23:2008–2014. doi: 10.1161/01.ATV.0000096655.56262.56. [DOI] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Miranda E, Weinl C, Harmer E, Holt CE. B-type Eph receptors ephrins induce growth cone collapse through distinct intracellular pathways. J. Neurobiol. 2003;57:323–336. doi: 10.1002/neu.10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Mao W, Luis E, Ross S, Silva J, Tan C, Crowley C, Chui C, Franz G, Senter P, Koeppen H, Polakis P. EphB2 as a therapeutic antibody drug target for the treatment of colorectal cancer. Cancer Res. 2004;64:781–788. doi: 10.1158/0008-5472.can-03-1047. [DOI] [PubMed] [Google Scholar]
- Margolis SS, Salogiannis J, Lipton DM, Mandel-Brehm C, Wills ZP, Mardinly AR, Hu L, Greer PL, Bikoff JB, Ho HY, Soskis MJ, Sahin M, et al. EphBmediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell. 2010;143:442–455. doi: 10.1016/j.cell.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston DJ, Dickinson S, Nobes CD. Rac-dependent trans-endocytosis of ephrinBs regulates Eph–ephrin contact repulsion. Nat. Cell Biol. 2003;5:879–888. doi: 10.1038/ncb1044. [DOI] [PubMed] [Google Scholar]
- Masood R, Xia G, Smith DL, Scalia P, Still JG, Tulpule A, Gill PS. Ephrin B2 expression in Kaposi sarcoma is induced by human herpesvirus type 8: phenotype switch from venous to arterial endothelium. Blood. 2005;105:1310–1318. doi: 10.1182/blood-2004-03-0933. [DOI] [PubMed] [Google Scholar]
- Meyer S, Hafner C, Guba M, Flegel S, Geissler EK, Becker B, Koehl GE, Orso E, Landthaler M, Vogt T. Ephrin-B2 overexpression enhances integrin-mediated ECM-attachment migration of B16 melanoma cells. Int. J. Oncol. 2005;27:1197–1206. [PubMed] [Google Scholar]
- Miao H, Strebhardt K, Pasquale EB, Shen TL, Guan JL, Wang B. Inhibition of integrin-mediated cell adhesion but not directional cell migration requires catalytic activity of EphB3 receptor tyrosine kinase. Role of Rho family small GTPases. J. Biol. Chem. 2005;280:923–932. doi: 10.1074/jbc.M411383200. [DOI] [PubMed] [Google Scholar]
- Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, et al. EphA2 mediates ligand-dependent inhibition ligand-independent promotion of cell migration invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Adamis AP, Shima DT, D’Amore PA, Moulton RS, O’Reilly MS, Folkman J, Dvorak HF, Brown LF, Berse B, et al. Vascular endothelial growth factor/vascular permeability factor is temporally spatially correlated with ocular angiogenesis in a primate model. Am. J. Pathol. 1994;145:574–584. [PMC free article] [PubMed] [Google Scholar]
- Mohle R, Green D, Moore MA, Nachman RL, Rafii S. Constitutive production thrombin-induced release of vascular endothelial growth factor by human megakaryocytes platelets. Proc. Natl. Acad. Sci. U.S.A. 1997;94:663–668. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai KK, Pasquale EB. ‘Eph’ective signaling: forward, reverse crosstalk. J. Cell Sci. 2003;116:2823–2832. doi: 10.1242/jcs.00625. [DOI] [PubMed] [Google Scholar]
- Murai KK, Pasquale EB. New exchanges in eph-dependent growth cone dynamics. Neuron. 2005;46:161–163. doi: 10.1016/j.neuron.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat. Rev. Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat. Cell Biol. 2005;7:270–277. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- Noren NK, Pasquale EB. Eph receptor–ephrin bidirectional signals that target Ras Rho proteins. Cell. Signal. 2004;16:655–666. doi: 10.1016/j.cellsig.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Noren NK, Pasquale EB. Paradoxes of the EphB4 receptor in cancer. Cancer Res. 2007;67:3994–3997. doi: 10.1158/0008-5472.CAN-07-0525. [DOI] [PubMed] [Google Scholar]
- Noren NK, Foos G, Hauser CA, Pasquale EB. The EphB4 receptor suppresses breast cancer cell tumorigenicity through an Abl–Crk pathway. Nat. Cell Biol. 2006;8:815–825. doi: 10.1038/ncb1438. [DOI] [PubMed] [Google Scholar]
- Ogita H, Kunimoto S, Kamioka Y, Sawa H, Masuda M, Mochizuki N. EphA4-mediated Rho activation via Vsm-RhoGEF expressed specifically in vascular smooth muscle cells. Circ. Res. 2003;93:23–31. doi: 10.1161/01.RES.0000079310.81429.C8. [DOI] [PubMed] [Google Scholar]
- Palmer A, Zimmer M, Erdmann KS, Eulenburg V, Porthin A, Heumann R, Deutsch U, Klein R. EphrinB phosphorylation reverse signaling: regulation by Src kinases PTP-BL phosphatase. Mol. Cell. 2002;9:725–737. doi: 10.1016/s1097-2765(02)00488-4. [DOI] [PubMed] [Google Scholar]
- Pasquale EB. Eph receptors ephrins in cancer: bidirectional signalling beyond. Nat. Rev. Cancer. 2010;10:165–180. doi: 10.1038/nrc2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitulescu ME, Adams RH. Eph/ephrin molecules—a hub for signaling endocytosis. Genes Dev. 2010;24:2480–2492. doi: 10.1101/gad.1973910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. The von Hippel-Lindau tumor suppressor, hypoxiainducible factor-1 (HIF-1) degradation, cancer pathogenesis. Semin. Cancer Biol. 2003;13:83–89. doi: 10.1016/s1044-579x(02)00103-7. [DOI] [PubMed] [Google Scholar]
- Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development. 2005;132:4097–4106. doi: 10.1242/dev.01971. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- Ruhe JE, Streit S, Hart S, Wong CH, Specht K, Knyazev P, Knyazeva T, Tay LS, Loo HL, Foo P, Wong W, Pok S, et al. Genetic alterations in the tyrosine kinase transcriptome of human cancer cell lines. Cancer Res. 2007;67:11368–11376. doi: 10.1158/0008-5472.CAN-07-2703. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev. 2002;16:2684–2698. doi: 10.1101/gad.242002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O’Connell S, Cowan CW, Hu L, Goldberg JL, et al. Ephdependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Salvucci O, de la Luz Sierra M, Martina JA, McCormick PJ, Tosato G. EphB2 EphB4 receptors forward signaling promotes SDF-1-induced endothelial cell chemotaxis branching remodeling. Blood. 2006;108:2914–2922. doi: 10.1182/blood-2006-05-023341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci O, Maric D, Economopoulou M, Sakakibara S, Merlin S, Follenzi A, Tosato G. EphrinB reverse signaling contributes to endothelial mural cell assembly into vascular structures. Blood. 2009;114:1707–1716. doi: 10.1182/blood-2008-12-192294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron- Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Sawamiphak S, Ritter M, Acker-Palmer A. Preparation of retinal explant cultures to study ex vivo tip endothelial cell responses. Nat. Protoc. 2010a;5:1659–1665. doi: 10.1038/nprot.2010.130. [DOI] [PubMed] [Google Scholar]
- Sawamiphak S, Seidel S, Essmann CL, Wilkinson GA, Pitulescu ME, Acker T, Acker-Palmer A. Ephrin-B2 regulates VEGFR2 function in developmental tumour angiogenesis. Nature. 2010b;465:487–491. doi: 10.1038/nature08995. [DOI] [PubMed] [Google Scholar]
- Scehnet JS, Ley EJ, Krasnoperov V, Liu R, Manchanda PK, Sjoberg E, Kostecke AP, Gupta S, Kumar SR, Gill PS. The role of Ephs, Ephrins, growth factors in Kaposi sarcoma implications of EphrinB2 blockade. Blood. 2009;113:254–263. doi: 10.1182/blood-2008-02-140020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schruefer R, Sulyok S, Schymeinsky J, Peters T, Scharffetter-Kochanek K, Walzog B. The proangiogenic capacity of polymorphonuclear neutrophils delineated by microarray technique by measurement of neovascularization in wounded skin of CD18- deficient mice. J. Vasc. Res. 2006;43:1–11. doi: 10.1159/000088975. [DOI] [PubMed] [Google Scholar]
- Schultz GS, Wysocki A. Interactions between extracellular matrix growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Segura I, Essmann CL, Weinges S, Acker-Palmer A. Grb4 GIT1 transduce ephrinB reverse signals modulating spine morphogenesis synapse formation. Nat. Neurosci. 2007;10:301–310. doi: 10.1038/nn1858. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of metabolism by hypoxia-inducible factor 1. Cold Spring Harb. Symp. Quant. Biol. 2011 Jul 22; doi: 10.1101/sqb.2011.76.010678. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, Greenberg ME. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone MA, Jr, Anderson DJ. Expression of ephrinB2 identifies a stable genetic difference between arterial venous vascular smooth muscle as well as endothelial cells, marks subsets of microvessels at sites of adult neovascularization. Dev. Biol. 2001;230:139–150. doi: 10.1006/dbio.2000.9957. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b +Gr1+ myeloid cells. Nat. Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA. Oxygen-induced retinopathy in the mouse. Invest. Ophthalmol. Vis. Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Smith FM, Vearing C, Lackmann M, Treutlein H, Himanen J, Chen K, Saul A, Nikolov D, Boyd AW. Dissecting the EphA3/Ephrin-A5 interactions using a novel functional mutagenesis screen. J. Biol. Chem. 2004;279:9522–9531. doi: 10.1074/jbc.M309326200. [DOI] [PubMed] [Google Scholar]
- Stein E, Schoecklmann H, Daniel TO. Eph family receptors ligands in vascular cell targeting assembly. Trends Cardiovasc. Med. 1997;7:329–334. doi: 10.1016/S1050-1738(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Steinle JJ, Meininger CJ, Forough R, Wu G, Wu MH, Granger HJ. Eph B4 receptor signaling mediates endothelial cell migration proliferation via the phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 2002;277:43830–43835. doi: 10.1074/jbc.M207221200. [DOI] [PubMed] [Google Scholar]
- Steinle JJ, Meininger CJ, Chowdhury U, Wu G, Granger HJ. Role of ephrin B2 in human retinal endothelial cell proliferation migration. Cell. Signal. 2003;15:1011–1017. doi: 10.1016/s0898-6568(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Stenzel D, Lundkvist A, Sauvaget D, Busse M, Graupera M, van der Flier A, Wijelath ES, Murray J, Sobel M, Costell M, Takahashi S, Fassler R, et al. Integrin-dependent -independent functions of astrocytic fibronectin in retinal angiogenesis. Development. 2011;138:4451–4463. doi: 10.1242/dev.071381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation vessel branching. Proc. Natl. Acad. Sci. U.S.A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kamo T, Ota S, Sugimura H. Association of Dishevelled with Eph tyrosine kinase receptor ephrin mediates cell repulsion. EMBO J. 2003;22:847–858. doi: 10.1093/emboj/cdg088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Ohashi R, Nakamura R, Shinmura K, Kamo T, Sakai R, Sugimura H. Tiam1 mediates neurite outgrowth induced by ephrin-B1 EphA2. EMBO J. 2004;23:1075–1088. doi: 10.1038/sj.emboj.7600128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Chen B, Lin T, Li Z, Pardo C, Pampo C, Chen J, Lien CL, Wu L, Ai L, Wang H, Yao K, et al. Restraint of angiogenesis by zinc finger transcription factor CTCF-dependent chromatin insulation. Proc. Natl. Acad. Sci. U.S.A. 2011;108:15231–15236. doi: 10.1073/pnas.1104662108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Toth J, Cutforth T, Gelinas AD, Bethoney KA, Bard J, Harrison CJ. Crystal structure of an ephrin ectodomain. Dev. Cell. 2001;1:83–92. doi: 10.1016/s1534-5807(01)00002-8. [DOI] [PubMed] [Google Scholar]
- Vihanto MM, Vindis C, Djonov V, Cerretti DP, Huynh-Do U. Caveolin-1 is required for signaling membrane targeting of EphB1 receptor tyrosine kinase. J. Cell Sci. 2006;119:2299–2309. doi: 10.1242/jcs.02946. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction angiogenic interaction between embryonic arteries veins revealed by ephrin-B2 its receptor Eph- B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010a;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, et al. Ephrin- B2 controls VEGF-induced angiogenesis lymphangiogenesis. Nature. 2010b;465:483–486. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- Wybenga-Groot LE, Baskin B, Ong SH, Tong J, Pawson T, Sicheri F. Structural basis for autoinhibition of the Ephb2 receptor tyrosine kinase by the unphosphorylated juxtamembrane region. Cell. 2001;106:745–757. doi: 10.1016/s0092-8674(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Xia G, Kumar SR, Masood R, Zhu S, Reddy R, Krasnoperov V, Quinn DI, Henshall SM, Sutherland RL, Pinski JK, Daneshmand S, Buscarini M, et al. EphB4 expression biological significance in prostate cancer. Cancer Res. 2005;65:4623–4632. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- Xia G, Kumar SR, Stein JP, Singh J, Krasnoperov V, Zhu S, Hassanieh L, Smith DL, Buscarini M, Broek D, Quinn DI, Weaver FA, et al. EphB4 receptor tyrosine kinase is expressed in bladder cancer provides signals for cell survival. Oncogene. 2006;25:769–780. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]
- Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 cytoskeletal regulators mediates axon pruning. Nat. Neurosci. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zagoura D, Keck C, Pietrowski D. Expression of Eph receptor tyrosine kinases their ligands in human Granulosa lutein cells human umbilical vein endothelial cells. Exp. Clin. Endocrinol. Diabetes. 2006;114:590–595. doi: 10.1055/s-2006-950499. [DOI] [PubMed] [Google Scholar]
- Xu Z, Jin H, Qian Q. Humanized anti-EphB4 antibodies for the treatment of carcinomas vasculogenesis-related diseases. Expert Opin. Ther. Pat. 2009;19:1035–1037. doi: 10.1517/13543770902835525. [DOI] [PubMed] [Google Scholar]
- Yang NY, Pasquale EB, Owen LB, Ethell IM. The EphB4 receptor-tyrosine kinase promotes the migration of melanoma cells through Rho-mediated actin cytoskeleton reorganization. J. Biol. Chem. 2006;281:32574–32586. doi: 10.1074/jbc.M604338200. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Rago C, Kinzler KW, Vogelstein B. Identification classification of p53-regulated genes. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14517–14522. doi: 10.1073/pnas.96.25.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Jin YT, Lin MT. Expression of Tie-2, angiopoietin-1, angiopoietin-2, ephrinB2 EphB4 in pyogenic granuloma of human gingiva implicates their roles in inflammatory angiogenesis. J. Periodontal Res. 2000;35:165–171. doi: 10.1034/j.1600-0765.2000.035003165.x. [DOI] [PubMed] [Google Scholar]
- Yuan K, Hong TM, Chen JJ, Tsai WH, Lin MT. Syndecan-1 up-regulated by ephrinB2/EphB4 plays dual roles in inflammatory angiogenesis. Blood. 2004;104:1025–1033. doi: 10.1182/blood-2003-09-3334. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion? J. Clin. Invest. 1997;99:2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bellingard V, Feng KT, McMaster M, Fisher SJ. Human cytotrophoblasts promote endothelial survival vascular remodeling through secretion of Ang2, PlGF, VEGF-C. Dev. Biol. 2003;263:114–125. doi: 10.1016/s0012-1606(03)00449-4. [DOI] [PubMed] [Google Scholar]
- Zhuang G, Brantley-Sieders DM, Vaught D, Yu J, Xie L, Wells S, Jackson D, Muraoka-Cook R, Arteaga C, Chen J. Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res. 2010;70:299–308. doi: 10.1158/0008-5472.CAN-09-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M, Palmer A, Kohler J, Klein R. EphB–ephrinB bi-directional endocytosis terminates adhesion allowing contact mediated repulsion. Nat. Cell Biol. 2003;5:869–878. doi: 10.1038/ncb1045. [DOI] [PubMed] [Google Scholar]
- Zisch AH, Pazzagli C, Freeman AL, Schneller M, Hadman M, Smith JW, Ruoslahti E, Pasquale EB. Replacing two conserved tyrosines of the EphB2 receptor with glutamic acid prevents binding of SH2 domains without abrogating kinase activity biological responses. Oncogene. 2000;19:177–187. doi: 10.1038/sj.onc.1203304. [DOI] [PubMed] [Google Scholar]