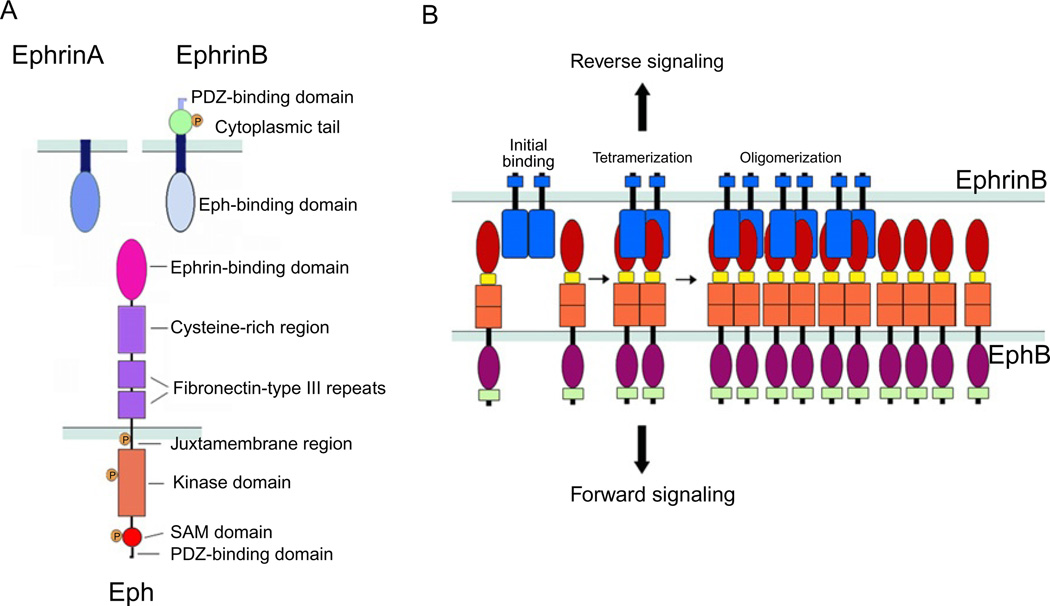
Fig. 1.
(A) Schematic representation of the domain structure and binding interfaces of Ephrins and Eph receptors. EphrinA ligands are attached to the cell surface through a glycosylphosphatidylinositol (GPI)-anchor; the extracellular domain contains an Eph receptor-binding domain that is connected to the transmembrane segment. EphrinB ligands are transmembrane proteins with an extracellular Eph receptor-binding domain connected to a transmembrane segment, which is followed by a short intracellular domain. The Eph receptors include an extracellular domain composed of an Ephrin-binding domain, a cysteine-rich segment that contains an epidermal growth factor (EGF)-like motif, and two fibronectin-type III domains; and a cytoplasmic region that contains a juxtamembrane region, the kinase domain, a sterile a-motif (SAM), and a binding site for PDZ-containing proteins. (B) Representation of initial binding of cell surface Eph and Ephrin molecules to form heterotetramers, which initiate signaling, and subsequent oligomerization to form large receptor/ligand clusters that expand laterally through hemophilic interactions between Eph receptors.