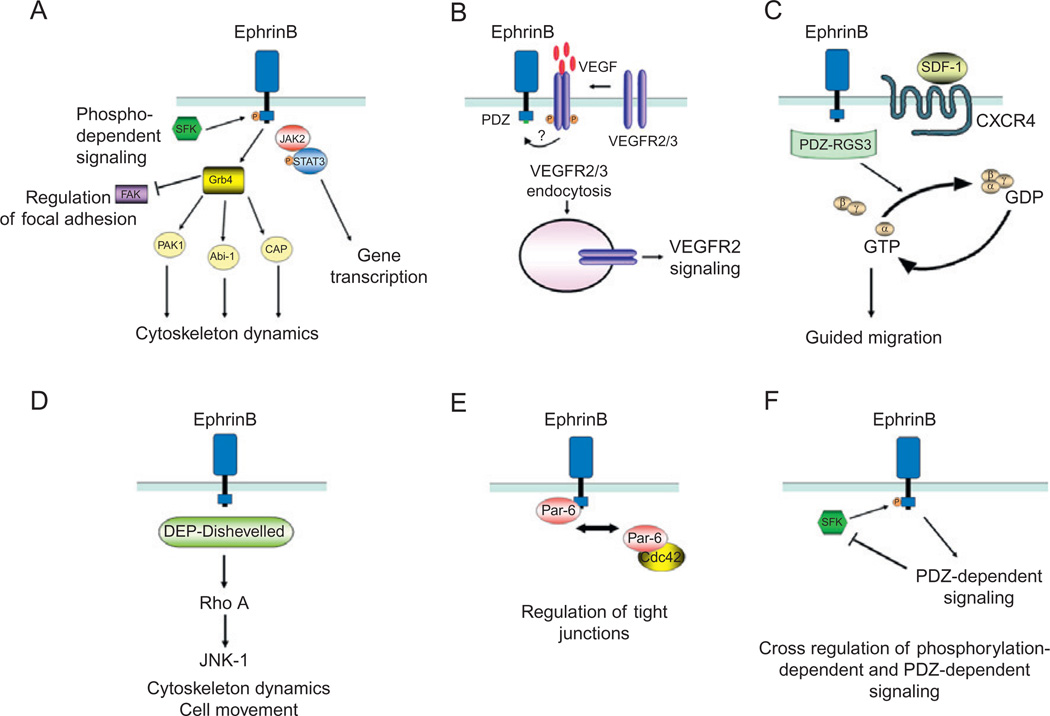
Fig. 3.
Mechanisms of EphrinB reverse signaling. (A) EphrinB activation by EphB receptor leads to the recruitment of Src family kinases (SFKs) that phosphorylate EphrinB intracellular domain at tyrosine residues. The adaptor molecule Grb4, which contains a Src-homology-2 (SH2) domain, is recruited to the phosphorylated EphrinB and initiates a number of signaling events that regulate cytoskeleton dynamics and focal adhesions. The phosphorylated EphrinB can also recruit the Jak2/STAT3 complex; the phosphorylated STAT3 translocates to the nucleus regulating expression of target genes. (B) Activated EphrinB regulates the internalization of VEGFR2 and VEGFR3 through PDZ-mediated binding of yet unknown protein(s). After VEGF binding, VEGFR is phosphorylated; signaling from activated VEGFR requires internalization, which is positively regulated by EphrinB signaling. (C) RGS3 is a PDZ-containing protein that constitutively binds to EphrinB and links signaling from the G-protein-coupled receptor CXCR4 to EphrinB. CXCR4 signaling in response to the ligand SDF1 is induced by the dissociation of Gβγ and GTP-Gα subunits. PDZ-RGS3 can inhibit CXCR4 signaling by enhancing the GTPase activity of the Gα subunit, resulting in the reformation of the inactive heterodimeric CXCR4 receptor. (D) The scaffold protein Dishevelled binds EphrinB through its DEP domain and mediates signaling via the Rho small GTPase pathway. (E) The scaffold protein Par6 associates with EphrinB resulting in the loss of tight junctions. PAR6 forms a complex with activated apical protein kinase C (aPKC) and Cdc42-GTP; the complex localizes to the apical cell junctions where it regulates tight junctions. EphrinB1 can compete with Cdc42 for binding to Par6 and thus reduce tight junctions. (F) Cross-regulation between EphrinB phosphorylation- and PDZ-dependent signaling pathways. The phosphatase PTB-BL, recruited through its PDZ domain to EphrinB cytoplasmic domain, inactivates Src and dephosphorylates EphrinB.