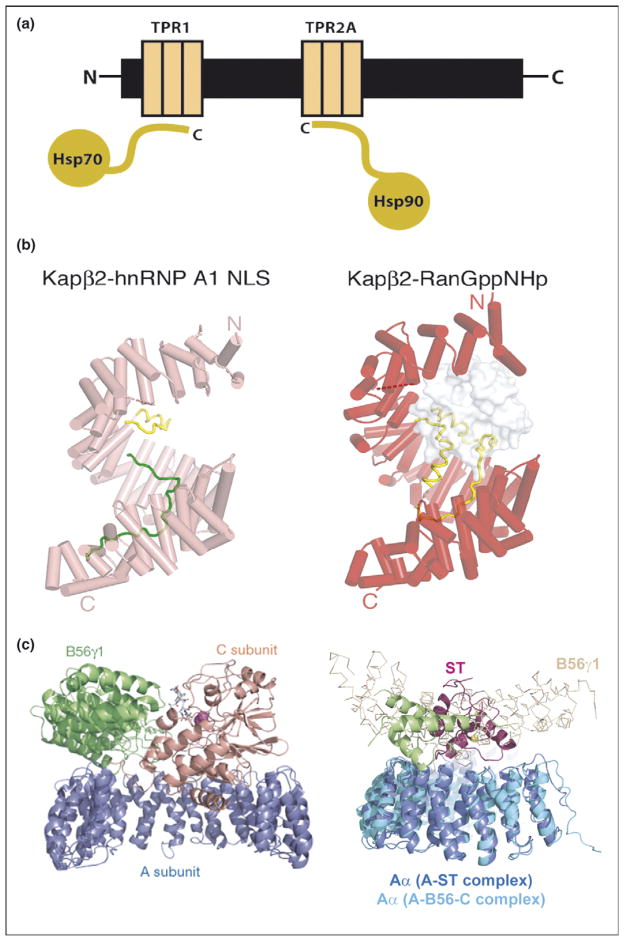
Figure 3.
Repeat proteins as multi-protein complex organizers. (a) Schematic representation of Hsp90/Hsp70 organizing protein (HOP) showing how two independent TPR domains serve to assemble the multi-chaperone complex. The figure shows the interactions between the TPR1 and TPR2A domain of HOP with the C-terminal sequences of Hsp70 and Hsp90, respectively. (b) Heat repeats of karyopherins present different binding surfaces for multi-protein binding. Structure of karyopherin Kapβ2 bound to NLS substrate (PDB ID: 1ot8) (left panel) and bound to the regulatory protein Ran (PDB ID: 1qbk) (right panel). The helices are represented as cylinders (pink in susbtrate-bound structure and red in the Ran-bound structure); structurally disordered loops are shown as dashed lines and HEAT repeat 8 (H8) loops are in yellow. The substrate hnRNP A1-NLS is shown as a green ribbon and the Ran protein as a surface representation in gray [10••]. (c) Protein phosphatase 2A (PP2A) HEAT repeats as scaffolding subunit. Left panel: Ribbon representation of the heterotrimeric PP2A holoenzyme crystal structure. The scaffolding subunit A colored in blue binds the regulatory subunit B56γ1 (green) and the catalytic subunit C (orange). Right panel: SV 40 Small T antigen competes with the regulatory subunit B. Structural superimposition of the A-ST complex (PDB ID: 2pf4) and the A-B56-C PP2A holoenzyme complex (PDB ID: 2iae). The two complexes are superimposed using A subunit HEAT repeats 2–10. The J and unique domain of ST are colored green and pink, respectively. The Cα trace of B56γ1 regulatory subunit are shown in light orange. It is clear that ST and B56γ1 bind to the same sites on PP2A A subunit [16••,17••].